1 Introduction
When shared resources are limited, the coexistence of closely-related and morphologically similar species is expected to create competitive interaction that may lead to niche partitioning [1]. Niche partitioning may induce the exploitation of different food resources, and sometimes favors the evolution of new phenotypes to exploit these resources, this phenomenon is known as “ecological character displacement” [1–4]. In the lakes of coastal British Columbia, sympatric stickleback species have diverged by habitat, i.e. limnetic vs. benthic, which allowed for the exploitation of different resources, i.e. planktonic vs. benthic prey, and led to morphological divergences, e.g., mouth size and gill rakers number [5]. The introduction of Darwin's finch species to a Galapagos island rapidly led to a divergence in the beak size of the native species, allowing them to exploit different resources than the invader [6]. Species may also evolve in different directions in allopatry because of local environmental conditions. Then, when the species enter in contact after range expansion, they have a sufficient level of morphological divergence allowing the exploitation of different resources and to coexist [7]. High niche overlap and morphological convergence may also occur when the resource is not limited or is non-substitutable [8–10]. In any case, the understanding of such niche partitioning (or its absence) among similar, related sympatric species allows for assessment of their distinct (or similar) roles in the ecosystem which is of particular importance when we are to assess the functional redundancy or, at the contrary, the functional complementarity of communities reflecting the vulnerability or resilience of ecosystem processes [11]. Several studies did highlight that the functional redundancy of various closely-related and morphologically similar reef fishes species was first overestimated, these species occupying distinct ecological niches and filling distinct functional roles on the reef [11–15].
In the Gulf of California (GC, Mexico), the damselfishes (Pomacentridae) are among the most conspicuous reef-fishes families [16–19]. Two species of the Stegastes genus are commonly seen in the GC, the beaubrummel gregory damselfish Stegastes flavilatus and the Cortez damselfish Stegastes rectifraenum. Stegastes flavilatus and S. rectifraenum look morphologically and ecologically similar and have been described as being active at the same time and over similar areas, suggesting the occurrence of possible competitive interactions [20]. Stegastes rectifraenum is an endemic species of the GC (latitude range: 22°–32° N) while S. flavilatus is also found in more tropical waters, with the GC representing its most northern distribution (latitude range: 4°S–33°N) [21]. Many damselfishes, and specially the genus Stegastes, are described as farmers [22]. This unique behavior within all teleosts involves the active management of a small garden of filamentous algae. Farming damselfishes maintain small-size (∼2 m2) crops of filamentous algae by chasing away intruders, such as other herbivorous fishes (e.g., Acanthuridae, Scaridae) or invertebrates (e.g., sea urchins) and weeding out undesirable algae [22]. Farmers feed on the cropped filamentous algae but also on the small invertebrates living on or within the mat of algae [22]. This behavior shapes the reefscape because it creates distinct algae and invertebrates communities and increases the productivity within the farms [23–25]. Because of the abundance of these species on shallow reefs [26] and their influence on the environment, the farming behavior may be a key element in reef ecosystem functioning. Therefore, it is essential to have a good understanding of the ecological niche of these species and their level of redundancy. Stegastes rectifraenum has been defined as a farming species [27], and while the situation is unknown for S. flavilatus, both species actively defend their territory during and out of their reproductive period [28]. Stegastes rectifraenum selectively feeds on filamentous algae [20,27,29], and a mix of benthic organisms have been found in their stomach contents [30]. This omnivorous diet seems to be associated with specific morphological characters such as a thick skull osteology and a single range of incisor premaxillar teeth for herbivory but a relatively short intestine and intestinal convulations more related to zooplanktivory [31]. The trophic ecology of S. flavilatus has been poorly studied but external observations suggest their behavior is similar to S. rectifraenum [20,28].
The goal of this study is to compare the distribution and the eco-morphological features of S. flavilatus and S. rectifraenum. We first investigated the spatial distribution of these two species within the GC and explored the potential effects of environmental variables on their abundance. Then, we studied the morphology of the two species through morpho-functional traits and geometric morphometric analyses. Morphological attributes may be good ecological indicators because they are expected to enhance the ability of the fish to perform key tasks such as feeding and locomotion [32]. Finally, we used stable isotope analysis to compare the trophic niche of the two species. We asked whether the level of ecological similarities between the two Stegastes species is consistent with the hypothesis of niche partitioning.
2 Materials and methods
2.1 Biogeographical distribution and effect of environmental variables
We gathered data from 341 scuba-diving censuses of 100 m2 (25 × 4 m) from 42 sites (8 ± 4 transects by site) in the GC (Fig. 1A, Appendix 1). Censuses were made in a depth range of 2 m to 28 m. For each census, we monitored the number of individuals of S. flavilatus and S. rectifraenum, and various environmental variables: (1) the maximal depth measured within a census, (2) temperature, (3) rugosity, and (4) substratum. The rugosity measures the topographic complexity, which was estimated by the ratio between the distance measured following the bottom contour and the linear distance (“chain link method” [33]). The substratum was described according to the proportion of seven categories: (1) unvegetated sand, (2) bare rock, (3) filamentous algae, (4) macroalgae, (5) corals, (6) dead bivalves’ shells, and (7) coralline algae. The type of substratum was recorded every 25 cm along with the 25 m-long censuses (100 points).
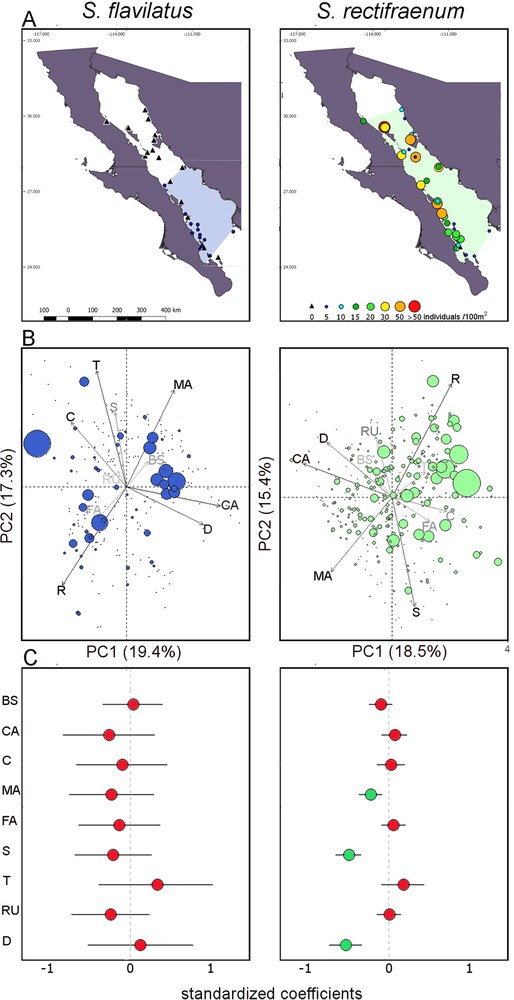
Biogeographical distribution and effect of environmental variables. (A) Distribution of the species in the Gulf of California. Colored-polygons on the maps encompass all the sites where both species were recorded. (B) Principal Component Analyses made on the environmental variables. The size of the circles is proportional to the amount of individuals, different scales have been used for both species because of the high difference in their abundance. The colors of the variables labels and arrows are according to their contribution. (C) Results of the Generalized Linear Mixed Effect Models. The mean effects of substratum, depth, rugosity, and temperature on the density of both species are shown. Values of independent variables have been standardized. Circles represent mean parameter estimates with their 95% confidence intervals. Green circles indicate significant mean values, while red circles indicate non significant mean values. BS: Bivalves’ shells; C: Coral; CA: Coralline algae; D: depth; FA: Filamentous algae; MA: Macroalgae; R: Rock; RU: rugosity; S: Sand; T: Temperature. For S. flavilatus, the analyses were run considering the sites within its geographical distribution in the Gulf of California.
First, we studied the latitude effect on the abundance of both Stegastes species, by running a Generalized Linear Model (GLM), with a quasi-Poisson distribution to deal with overdispersion. As S. flavilatus was not observed beyond 28° N, we did not consider, for this species, sites beyond this latitude in the following analyses. Second, we ran a Principal Component Analysis (PCA), to visualize the relationships between the studied environmental variables and the abundances of both species. Third, we determined the relationships between the environmental variables and the abundance of both species by performing Generalized Linear Mixed Models (GLMMs). We assessed the collinearity of the variables by computing the “variance inflation factor” (VIF) with the vif function of the car R package [34]. In the total absence of collinearity, all variables should have a VIF value equal to one but, as a rule of thumb, values below five are acceptable [35]. We considered the sites as a random variable to control spatial-autocorrelation issues. Because Quasi-Poisson distribution is not available for mixed models, we chose a negative binomial distribution to deal with overdispersion. All analyses were run in the R software [36]. The GLMMs were run with the lme4 R package [37]. We checked spatial autocorrelation issues with the Moran's I test, using the ape R package [38].
2.2 Ecomorphological analyses
2.2.1 Specimen collection
We collected specimens in Cajelitas, a coastal reef of the GC at the proximity of the city of La Paz (24°21′14′′ N, 110°17′02′′ W), from March to May 2016. Specimens were captured using nets and were directly anesthetized on ice before being preserved in 96% ethanol. We collected 42 adult specimens (20 specimens of S. flavilatus, standard length = 83.8 ± 6.7 mm [mean ± SD]; 22 specimens of S. rectifraenum, standard length = 77.6 ± 7.2 mm).
2.2.2 Morphological analysis: geometric morphometrics and additional measures
The specimens were photographed with a right-lateral view using a digital camera. Then, two geometrical morphometric analyses were conducted through the x-, y-coordinates of 20 homologous landmarks and 14 semi-landmarks [4,39] using the software TPSDIG [40]. We first analyzed the shape of the cephalic region by 14 fixed landmarks (Fig. 2). Then, we analyzed the shape variation of the whole body considering eight fixed landmarks and 14 semi-landmarks (Fig. 2). The chosen LMs allows the capture of variation related to feeding and locomotion mechanics [41–43]. The semi-landmarks help to capture the curvature of the fish body. The generation of shape data followed Frédérich et al. [44]. We optimally aligned the specimens of each species using a Generalized Procrustes Analysis “GPA” [45]. GPA translates all specimens to the origin, scales them to unit-centroid size, and optimally rotates them until the corresponding points align as closely as possible. The resulting aligned Procrustes coordinates represent the shape of each specimen [46]. We then performed a relative warps analysis [47] on the consensus landmark configuration for each species to generate a morphospace illustrating the major axes of shape variation. We used the species scores on all individual warps for exploring the phenotypic difference, by performing Procrustes-Anova with the geomorph R package [46]. Procrustes-Anova uses permutation procedures to assess statistical hypotheses describing patterns of shape variation and covariation for a set of Procrustes shape variables [46].
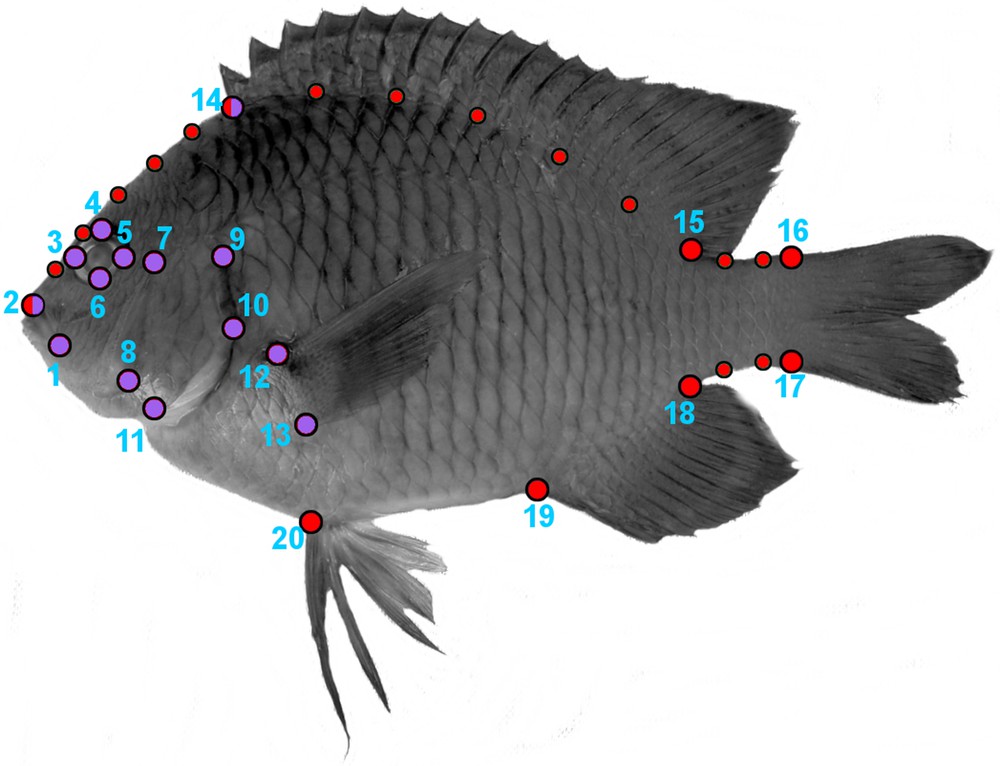
Landmarks configurations for geometric morphometric analysis. The red and purples landmarks were used to analyze the shape of the body and the cephalic region, respectively. Eight fixed landmarks (large size circles) and 14 semi-landmarks (small size circles) were selected for the body shape. Fourteen fixed landmarks were selected for the cephalic region. (1) Anterior tip of the snout (premaxilla), (2) posterior end of the maxilla, (3) anterior end of the eye, (4) upper end of the eye, (5) posterior end of the eye, (6) lower end of the eye, (7) antero-dorsal origin of the operculum, (8) postero-ventral corner of the preoperculum, (9) most supero-dorsal margin of the operculum, (10) posterior end of the operculum, (11) ventral extremity of suboperculum, (12) upper insertion of the pectoral fin, (13) lower insertion of the pectoral fin, (14) anterior and (15) posterior insertion of the dorsal fin, (16) dorsal and (17) ventral base of the caudal fin, (18) posterior and (19) anterior insertion of the anal fin, (20) insertion of the pelvic fin.
Two additional morphological traits directly linked to feeding behavior, i.e. the total mass of the adductor mandibulae (AM) and the number of gill rakers on the first branchial arch, were measured. The AM close the mouth in all teleosts [48], and their development has been associated with different trophic diets and/or feeding modes: species that need to scrape, excavate, or feeding on hard-shell prey have more developed AM than those feeding on small elusive prey such zooplanktivorous species [12,32,49]. The gill rakers are bony processes that project from the branchial arch (gill arch) and retain small organisms captured by suction. The gill rakers are generally found in higher number in zooplanktivorous species [50]. Dissections were made under a binocular microscope, and the AMs were weighed to the nearest 0.1 mg. The AM mass was collected for 19 S. flavilatus and 20 S. rectifraenum. Gill rakers information was collected on 18 S. flavilatus and 17 S. rectifraenum. The AM mass was adjusted by the standard length (SL) of the individuals following Hulsey et al. [51]. The mass of the muscle was cube rooted because mass generally scales with the third power of length. Then, we log-transformed the results to account for increased variance as measurements increase with body size. Finally, a linear regression was fitted between these mass-transformed data and the log of SL. As the SL had a significant effect on the AM mass (Estimate [± SE] = 1.25 ± 0.16. t-value = 8.03, P-values < 0.001), the residual values of the model were used for further comparisons. A t-test was then performed to compare the two Stegastes species. Visual inspection of the residuals did not reveal violations of the parametric assumptions.
Although the number of gill rakers was count data, a GLM with a Poisson distribution was not appropriate because of underdispersion. A Gaussian distribution was not adequate because of normality violation (high negative asymmetry of the residuals, skew = –0.87), and heterogeneity of the variance was not observed. We used, therefore, a non-parametric approach, and we performed a permutational t-test (999 iterations) with the R function t.perm [52].
2.2.3 Trophic habits
Samples of lateral muscle tissue (± 2 cm3) of the captured fish were used for stable isotope analysis (20 specimens of S. flavilatus and 22 of S. rectifraenum). To describe the isotopic niches (i.e. proxy of trophic niches) of these species in the studied area and compare it between them, we collected dominant producers and consumers on the reef. Three primary food sources (i.e. plankton, benthic invertebrates, and filamentous algae) were collected. Plankton was trapped using a net with a mesh of 250 μm, towed on the reef at a depth of 1 to 2 m. The reef had a maximal depth of ∼3–4 m, twelve trackings were made during two different days. Turf algae from rocks on four territories of Stegastes spp. were scrapped underwater with a knife. Turf samples were taken during four different days. Benthic small invertebrates (including mainly isopods, amphipods, copepods, annelids, and small decapods) were collected from the reef during the night using a light trap. The trap consisted of a can of water containing glowsticks (lit for 8–12 h), weighted to stay on the bottom. The content of the trap was then filtered with a 250-μm mesh. Six traps were used to collect benthic invertebrates during two different nights. Two representative and highly abundant species were selected as comparative species: the damselfish Abudefduf troschelii (28 specimens) and the wrasse (Labridae) Thalassoma lucasanum (29 specimens). Abudefduf troschelii is considered to feed mainly on elusive prey in the water column [28], and T. lucasanum is omnivorous, feeding on a mix of crustaceans and algae [20].
Samples of lateral muscle tissue and potential food sources were dried in the sun for several days before being ground into a homogeneous powder. Samples were protected by a double net with a mesh of 0.5 mm to avoid contamination. The south of the Gulf of California undergoes desert environmental conditions and the temperature in the sun easily rises to 40–50 °C, which is similar to a lab heater. All samples were analyzed for δ13C and δ15N [‰], via continuous flow – elemental analysis – isotope ratio mass spectrometry (CF-EA-IRMS) at the University of Liège (ULiège, Belgium), using a vario MICRO cube elemental analyzer (Elementar, Hanau, Germany) coupled with an IsoPrime100 mass spectrometer (Isoprime, Cheadle, United Kingdom). Sucrose (IAEA-C6; mean ± SD: δ13C = –10.8 ± 0.5‰) and ammonium sulfate (IAEA–N2; δ15N = 20.3 ± 0.2‰) were used as certified reference materials. Both reference materials calibrated against the international isotopic references, i.e. the Vienna Pee Dee Belemnite for carbon and atmospheric air for nitrogen. Routine measurements of internal laboratory standards (i.e. amphipods; glycine [Merck, Germany]) indicate a standard deviation of 0.3 ‰ for δ13C and δ15N.
We performed t-tests to compare δ13C and δ15N values of the two Stegastes species. Size effect was also investigated by linear regression. No parametric violations were observed. The stable isotope mixing model SIAR (Stable Isotope Analysis in R) was used to estimate the relative contribution of the different food sources to the diet of the fish species [53]. The mixing model included the isotopic composition of each individual, isotopic composition of food sources (expressed as mean ± SD), and trophic enrichment factors (TEFs, expressed as mean ± SD) that corresponded to the net isotopic composition change between a consumer and its ingested food source(s). For carbon, we had a unique TEF factor of 1.6 ± 0.5 ‰, which was already used in other studies [54,55] and is in the range of generally observed TEF for 13C in omnivorous fishes [56]. TEFs for nitrogen were adapted according to the food type potentially assimilated by the fish. For the turf algae source, we used a TEF of 5.1 ± 0.6 ‰, a TEF adapted for herbivorous fish [55,57]. For animal sources (i.e. zooplankton and benthic invertebrates), we used a classical TEF for nitrogen of 2.3 ± 0.5 ‰ [55,58]. The model was run with 5 × 105 iterations and burn-in size set as 5 × 104. Dietary contributions were represented by probability density plots with 95% confidence intervals. Finally, SIBER (Stable Isotope Bayesian Ellipses in R; [59]) was used to generate bivariate standard ellipses representing core isotopic niches of the four consumers.
3 Results
3.1 Biogeographical distribution
Stegastes rectifraenum was observed at all sites except one (81% of the censuses, Appendix 1). Stegastes flavilatus was much less common and was only observed at 24 sites (15% of the censuses). No S. flavilatus was observed at latitudes higher than 28° N (Fig. 1A). Stegastes rectifraenum was abundant in the whole GC, but its density increased (slightly) northwards (estimate = 0.10 ± 0.04, df = 1.339, t-value = 2.50, P-value = 0.013), while the abundance of S. flavilatus decreased along the latitude (estimate = –0.62 ± 0.15, df = 1.339, t-value = –4.13, P-value < 0.001). When considering only censuses where both species were present (46 censuses), S. rectifraenum was much more abundant than S. flavilatus (i.e. 24.96 ± 25.02 individuals per 100 m2, against 3.61 ± 3.43 for S. flavilatus, Fig. 1A). The visualization of the PCA did not highlight an abundance pattern for S. flavilatus along with the environmental variables (Fig. 1B). On the other hand, a pattern was visible for S. rectifraenum whose abundance seemed to decrease along with depth and coralline algae, macroalgae, and sand cover (Fig. 1B, Table 1). The variance inflation factor (VIF), which assesses the linearity among environmental variables, was high (> 10) for the following variables: bare rock, filamentous algae, macroalgae, and coralline algae (Table 2), with the latter three being logically negatively correlated with the first one (Fig. 1B, Table A1). Thus, we withdrew the bare rock variable from the model which considerably decreased the VIF (< 1.5 for all the variables, Table 2). The GLMMs supported the descriptive results of the PCA for S. flavilatus as no predictor had a significant relationship to the abundance of this species (Fig. 1C, Table A2). The PCA results were also supported for S. rectifraenum except for coralline algae. Macroalgae, sand, and depth had a negative relationship with the abundance of S. rectifraenum (Fig. 1C, Table A3). No spatial autocorrelation issues were found (Table A4).
Correlation of the environmental variables with the first two axes of the principal component analysis.
| Stegastes flavilatus | Stegastes rectifraenum | |||
| PC1 | PC2 | PC1 | PC2 | |
| Bivalves’ shells | 0.13 | 0.12 | –0.11 | 0.12 |
| Coralline algae | 0.57 | –0.09 | –0.55 | 0.17 |
| Coral | –0.34 | 0.30 | 0.34 | –0.07 |
| Macroalgae | 0.29 | 0.45 | –0.38 | –0.37 |
| Filamentous algae | –0.20 | –0.17 | 0.23 | –0.12 |
| Rock | –0.39 | –0.46 | 0.37 | 0.57 |
| Sand | –0.07 | 0.34 | 0.14 | –0.54 |
| Temperature | –0.18 | 0.54 | 0.20 | 0.13 |
| Rugosity | –0.05 | 0.07 | –0.10 | 0.30 |
| Depth | 0.47 | –0.18 | –0.40 | 0.28 |
Values of the “variance inflation factor” (VIF). VIF are indicated with the model considering all variables and the one withdrawing the variable bared rock (indicated by an asterisk).
| Variables | VIF | VIF |
| Depth | 1.13 | 1.12 |
| Rugosity | 1.06 | 1.04 |
| Temperature | 1.25 | 1.25 |
| Sand | 5.37 | 1.11 |
| Macroalgae | 13.65 | 1.19 |
| Filamentous algae | 20.79 | 1.32 |
| Bared rock* | 28.37 | — |
| Corals | 3.49 | 1.24 |
| Coralline algae | 10.84 | 1.27 |
| Bivalves’ shells | 2.03 | 1.07 |
3.2 Eco-morphology
Geometric morphometric analyses revealed distinct body and head shapes between S. flavilatus and S. rectifraenum (Table 3). The main body shape variation was a more rounded shape for S. flavilatus than for S. rectifraenum (Fig. 3A). Stegastes flavilatus had also a more robust cephalic shape with a shorter snout and a higher supraoccipital crest (Fig. 3A), this latter being located near the anterior insertion of the dorsal fin (Fig. 2). Stegastes flavilatus had more gill rakers than S. rectifraenum (t = 2.88, df = 1.33, 999 permutations P-value = 0.007, Fig. 3B). The AM mass did not differ between species (estimate = –0.012 ± 0.012, t-value = –0.985, df = 1.37, P-value = 0.331, Fig. 3C).
Morpho-geometrical comparisons in the head and body shapes of both Stegastes species. The results of the procrustes Anova made on the PC scores are showed.
| df | SS | Variance | R 2 | F | P-value | |
| Head shape | ||||||
| Species | 1 | 0.014 | 0.014 | 0.06 | 2.47 | 0.035 |
| Residuals | 42 | 0.243 | 0.006 | 0.94 | ||
| Total | 43 | 0.258 | ||||
| Body shape | ||||||
| Species | 1 | 0.007 | 0.007 | 0.09 | 4.42 | <0.001 |
| Residuals | 42 | 0.065 | 0.002 | 0.91 | ||
| Total | 43 | 0.072 |
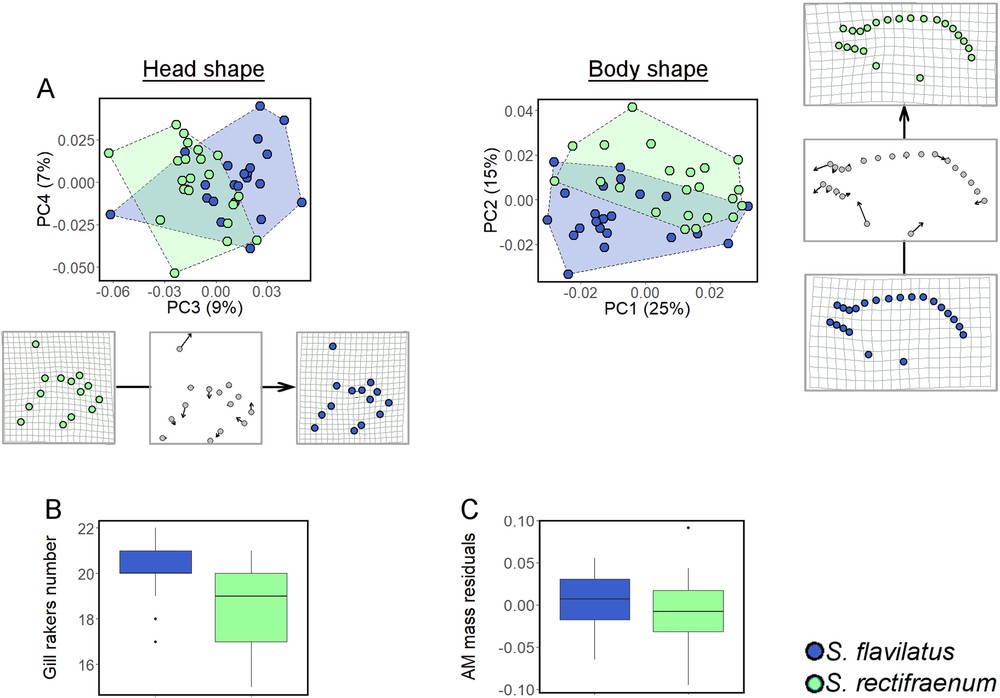
Morphological comparison between Stegastesflavilatus and S. rectifraenum. (A) Morpho-geometric analyses of the head and body shape. The axes of the Principal Component Analyses that best illustrated the separation between the two species are showed. The deformation grids represent the mean deformation along the PC3 and PC2 axes for the head and body shape, respectively. The transformations are also depicted as vectors of landmarks displacements to better illustrate the changes along the axes. (B) Number of gill rakers, (C) Size-corrected (residuals) mass of the adductor mandibulae. For B and C, median, 25th and 75th percentiles, and 95% confidence interval are showed.
Stegastes flavilatus had higher δ15N values than S. rectifraenum (estimate = 1.5 ± 0.24, t-value = 6.36, P-value < 0.001), and no differences were found for δ13C values (estimate = 0.17 ± 0.23, t-value = 0.76, P-value = 0.45) (Table 4). No overlap was observed between the standard ellipses of the two Stegastes species (Fig. 4A). The SIAR modeling output (Fig. 4B) indicated that pelagic food (plankton) is an important food source for both Stegastes species (S. flavilatus: mean = 39%, 95% credibility interval (CI) = 35–42%; S. rectifraenum: mean = 47%, 95% CI = 38–55%) (Fig. 4B). The two Stegastes diverged in the importance of zoobenthos and turf algae. The zoobenthos contribution is more important for S. flavilatus (mean = 48%, 95% CI = 41–55%) than S. rectifraenum (mean = 24%, 95% CI = 10-37%). While, the turf algae contribution is more important for S. rectifraenum (mean = 30%, 95% CI = 22–38%) compared to S. flavilatus (mean = 13%, 95% CI = 7–19%) (Fig. 4B). Neither of the two Stegastes species showed an overlap in their standard ellipses with A. troschelii (Fig. 4A), which is clearly a zooplanktivore (mean = 76%, 95% CI = 73–78%) (Fig. 4B). An important overlap in the standard ellipses was observed between S. rectifraenum and T. lucasanum (Fig. 4A), which relied on the three food sources in similar proportion (plankton: mean = 39%, 95% CI = 34-44%; zoobenthos: mean = 35%, 95% CI = 27-44%; turf algae: mean = 25%, 95% CI = 19-32%). No size effect was detected on δ13C and δ15N values for S. rectifraenum (δ13C: estimate = 0.01 ± 0.02, t-value = 0.67, P-value = 0.512; δ15N: estimate = 2.36 ± 2.51, t-value = 0.94, P-value = 0.36). While no size effect was detected on δ13C values for S. flavilatus (estimate = –0.06 ± 0.03, t-value = –1.8, P-value = 0.094), a significant effect was detected on δ15N values (estimate = 0.08 ± 0.02, t-value = 4.17, P-value < 0.001).
Isotopic signatures of the sources and the studied species.
| 13C (mean ± SD) | 15N (mean ± SD) | |
| Sources | ||
| Turf | –11.16 ± 0.31 | 3.19 ± 2.51 |
| Benthic invertebrates | –10.86 ± 1.26 | 12.17 ± 1.57 |
| Plankton | –18.94 ± 0.38 | 14.28 ± 1.16 |
| Species | ||
| Stegastes flavilatus | –12.27 ± 0.84 | 14.83 ± 0.72 |
| Stegastes rectifraenum | –12.44 ± 0.72 | 13.32 ± 0.81 |
| Abudefduf troschelii | –15.51 ± 0.36 | 14.50 ± 0.58 |
| Thalassoma lucasanum | –12.5 ± 1.15 | 13.69 ± 0.83 |
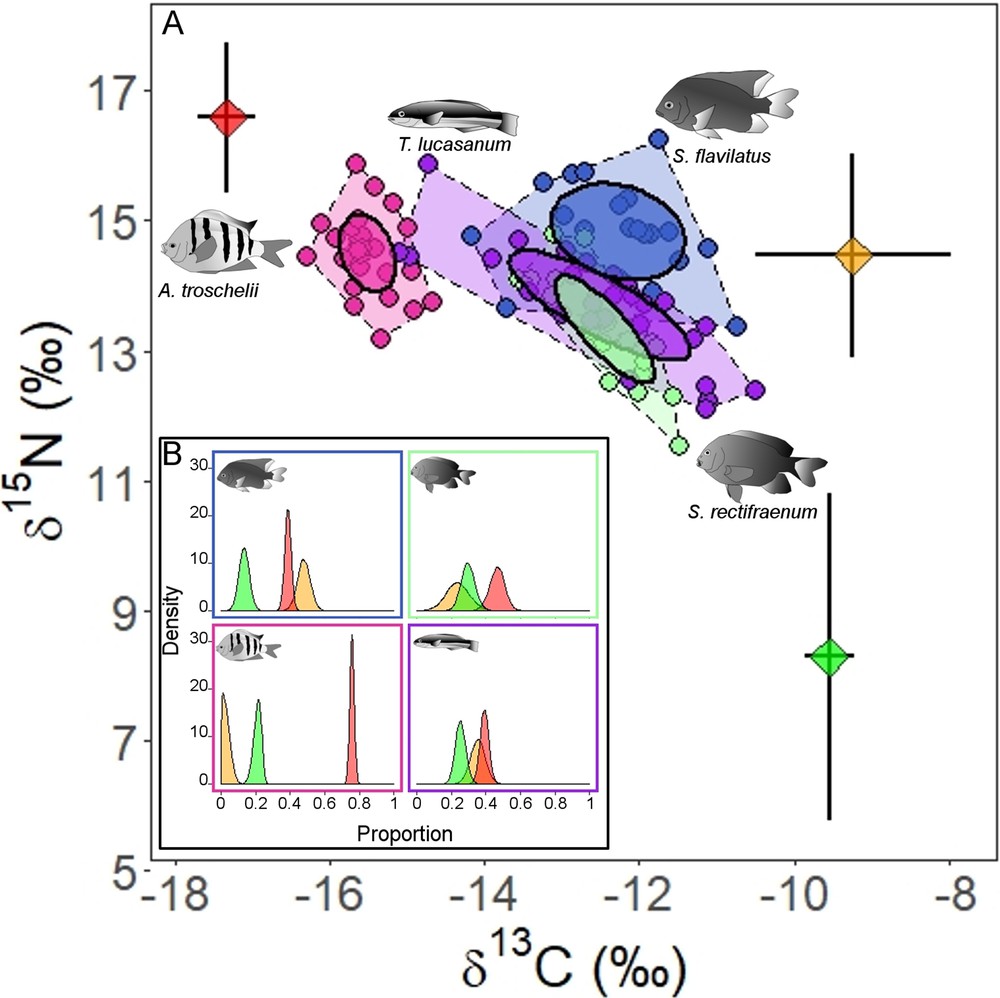
Isotopic niche of Stegastes flavilatus and S. rectifraenum. (A) Stable isotope bi-plot of the two Stegastes species and two other common reef fishes species (Abudefduf troschelii and Thalassoma lucasanum), added for comparison purposes. Convex hulls and standard ellipses are showed for each species. Diet sources values (mean ± SD) are color-plotted: red = plankton, orange = zoobenthos, and green = turf. The trophic enrichment factors have been added to the source values. B) Density plots of the relative contribution of the three food sources for the four fish species from the mixing model. The same color code as in (A) has been chosen.
4 Discussion
Stegastes rectifraenum is abundant throughout the entire GC, with a slight increase in abundance towards the north, while S. flavilatus has only been seen in the central and southern parts. The geographic distribution of S. flavilatus was thought to reach 33°N [21], but our current study, and two previous studies confirm its absence above 28°N [17,60]. Stegastes species are among the most conspicuous site-attached species of reefs; its presence is unlikely to pass unnoticed in the northern GC. Stegastes flavilatus is a tropical species and its northwards distribution is probably stopped by the changes in environmental conditions at the middle of the GC switching from tropical to temperate conditions [61,62]. The absence of S. flavilatus at high latitude could affect the abundance of S. rectifraenum that can increase northwards because of a reduction of competition. The GC represents one extreme of the geographical distribution of S. flavilatus and may represent suboptimal environmental conditions which could explain its low abundance. In contrast, S. rectifraenum is an endemic species of the GC and is likely to be more adapted to this region. The environmental niche of herbivorous damselfishes is partially led by the ecological requirement of their algal crop [63]. Depth, sand, and macroalgal cover were negatively correlated with the abundance of turf (filamentous algae; Table A1), an important food source for S. rectifraenum, and may explain the decrease in the densities of the species along with these three variables. Plankton and benthic invertebrates are less related with particular environments in reef ecosystems than filamentous algae [64]. Stegastes flavilatus distribution may be less influenced by the type of substratum and depth than S. rectifraenum due to its lower reliance on turf as a food source. The distribution of S. flavilatus may also be more shaped by the competitive interaction with S. rectifraenum than environmental variables. Future studies investigating regions where S. flavilatus is abundant and sometimes the dominant Stegastes species [65,66] should shed light on the relationship between environmental variables and the abundance of this species.
Damselfish are categorized into three trophic guilds [67]. The pelagic feeders that feed mainly on planktonic copepods; the benthic feeders that mainly graze on filamentous algae and/or benthic invertebrates; and an intermediate group that includes species that forage for their prey in pelagic and benthic environments in variable proportions (planktonic and benthic copepods, small vagile invertebrates, and filamentous algae) [67,68]. Stegastes flavilatus and S. rectifraenum belong to the subfamily Stegastinae, in which almost all species are considered to be benthic feeders and farming species [44,57,58,67,69]. Nevertheless, our study showed that although S. flavilatus and S. rectifraenum are not pelagic feeders like A. troschelii, plankton represents a third to a half of their assimilated food. Thus, both species should be referred to “intermediate feeders”, preying upon sources from benthic and pelagic environments [68]. These results challenge the traditional interpretation of the trophic ecology of farming species and support a recent study in Kimbe Bay (coral triangle, Indo-Pacific) showing that various farming damselfishes relied from a moderate to a high degree on planktonic food sources [70]. The trophic ecology of territorial damselfishes may also be geographically variable as the species Plectroglyphidon lacrymatus, was confirmed as a benthic feeder by its stomach content and stable isotope analyses along the coasts of Madagascar [67]. Yet, this species was found to feed, in equal proportions, on reef-based food sources (mainly turf and zoobenthos) and planktonic sources in the Kimbe Bay [70]. Our results, and those of Eurich and colleagues [70], highlight higher diversity in the trophic habit of farming damselfishes which do not rely solely on “gardened” food (filamentous algae or zoobenthos) in their territory, but also depend on external food sources. The degree of this dependency should be investigated by a large-scale study on farming damselfishes.
In the field, we did not observe Stegastes species feeding in the water column, unlike T. lucasanum (personal observation). However, a previous study did report S. rectifraenum feeding recurrently on feces from zooplanktivorous fish species (A. troschelii) directly in the water column but this behavior was not observed in S. flavilatus [28]. The importance of planktonic food for the two Stegastes spp. would suggest that the capture of zooplankton is a dominant foraging activity. Yet, both Stegastes spp. forage mainly within their territory [28] which would mean that they mainly prey upon the plankton brought by currents within their territory, instead of actively foraging in the water column.
Two previous studies using stomach content analyzes showed that S. rectifraenum was only feeding on filamentous algae and zoobenthos [27,30]. Stomach contents and stable isotopes analyses are complementary approaches with their limitations and strengths. Stomach contents are more specific than stable isotopes but they only reflect what the fish consumed during the few hours before the sampling. In addition, some preys are digested faster than others. For instance, very few zooplanktonic organisms have been found in the stomach contents of Chrysiptera annulata while stable isotope analysis revealed it was a major source of food [55]. Unidentified organic matter had been found in the stomach content of S. rectifraenum and we cannot exclude it contented digested zooplanktonic preys [30]. Another hypothesis involves a spatial and/or temporal variation in the feeding habit of S. rectifraenum. The two previous studies on the feeding habits of S. rectifraenum were carried out on a rocky reef, around 140 km south of the coral reef in our study, and further investigation should highlight if such trophic variation is supported.
Detritus is commonly found in the stomach content of damselfishes and can represent up to 30% of the assimilated food [58]. This detritus may include organic matter of animal origin, such as fish feces and corals mucus, with captured animal or animal organic debris [55]. This source of animal origin was not taken into account in our study, nor in the study of Eurich and colleagues [70], and it could also represent an important contribution. Thus, we must accept that, by not considering this potential food source, we may have introduced a bias in our estimation of the relative contribution of zoobenthos and zooplankton.
Although both Stegastes species are intermediate feeders that feed on pelagic and benthic prey, our results show that they differ in their feeding habits. Turf contributed the least to the assimilated food in S. flavilatus, which fed mainly on animal prey (Fig. 4B). Moreover, S. flavilatus had a higher trophic position than S. rectifraenum; with the larger individuals having the highest trophic position. This suggests that they may feed on larger animals, as it has been observed in other damselfish species [71]. Stegastes rectifraenum had an omnivorous diet, with both animal and vegetal organisms being important food sources. Stegastes rectifraenum occupied a very similar isotopic niche to the wrasse T. lucasanum, which is known to feed on plankton and small vagile invertebrates [21], as well on turf (Fig. 4B).
The trophic divergence between the two species may also be associated with the divergence in their morphology. The buccopharyngeal of a fish can be modeled as a truncate cone, whose small base is the circular opening of the mouth and large base is located behind the branchial basket on the level of operculum [72]. The higher supraoccipital crest of S. flavilatus in comparison to S. rectifraenum improves the design of the cone and allows the insertion of a well-developed epaxial muscle mass responsible for the rise of the neurocranium during mouth opening [72] which increases its suction force capability and makes the capture of mobile preys more efficient [73–75]. Furthermore, S. flavilatus had more gill rakers, which retain animal prey captured by suction. The higher robustness of the cephalic region in S. flavilatus may reflect more robust mouth structures and a higher ability to seize and crush bigger food items. Eventually, a study on pharyngeal jaw morphology showed a divergence in the dentition between S. flavilatus and S. rectifraenum; the former has molariform lower pharyngeal jaw teeth that may help to crush benthic organisms, while the latter has papilliform dentition, better suited to processing filamentous algae [76]. Stegastes rectifraenum has a more fusiform body shape that may provide higher swimming abilities than S. flavilatus, and would make S. rectifraenum to be more adapted to shallow reefs (< 10 m) directly exposed to wave actions as no crest are present in the reefs in the GC. High wave exposure favored the feeding function of more fusiform bodies in coral reef herbivory in the Palau Archipelago [77]. Nevertheless, no relationship between fish body fineness ratio (elongation) and the habitats or depths has been found for various damselfishes species in the Lizard Island [78]. For median-paired fin swimmers such as the Pomacentridae, the body fineness ratio only explains a small fraction of the variation in swimming performance observed across species [79]. The shape difference between the two species may also reflect a divergence on the way the food resource is extracted [28], S. rectifraenum, with its sharper head and longer snout has a higher degree of penetration and could more actively forage food within the filamentous algae matrix or crevices [80]. The fact that S. flavilatus do not feed on filamentous algae but mobile prey does not mean it is not a farming species. The crops of filamentous algae can also be managed to shelter benthic invertebrates on which S. flavilatus is feeding [22]. This farming activity may explain why S. flavilatus has AM as developed as S. rectifraenum. To maintain the crops, farmers exclude intruders such sea urchins by seizing them and depositing them outside the territory. Small rocks are also removed from the area and undesirable algae may be weeded out [22]. All these behaviors require forceful bite and well-developed AM.
Evidence of niche partitioning has been demonstrated in various other territorial damselfish species [70]. Some species had different spatial distributions on the reef or, when the distributions overlapped, a divergence in food source contributions was generally observed [70]. Nevertheless, two species, Pomacentrus bankanensis and Neoglyphidon nigroris, were neighbor species [81] and overlapped their isotopic space, with planktonic prey being their main food source. These two species foraged in the water column, an unusual foraging behavior for territorial and “farmer” damselfishes, but which likely reduced their interspecific competition. In our study, the captured specimens for isotopic and morphology came from a shallow reef where both species coexist in close proximity and where spatial segregation, or water column foraging, were not apparent (personal observation, but see [28]). Thus, the competition between both species may have driven the observed trophic niche partition and the evolution of different morphology to exploit the resources. On the other hand, the divergences may have evolved in allopatry. Indeed, both species adapted to different environmental conditions with S. flavilatus having more tropical affinities and S. rectifraenum being an endemic species from the GC. The evolution of distinct ecology and morphology in allopatry may have subsequently enabled coexistence when sympatry occurred following an extension of the geographical distribution of one or both species. Future studies should investigate the ecological niches and morphology of the two species in regions without geographical overlap, i.e. in the north of the GC for S. rectifraenum or outside the GC for S. flavilatus, to shed light on the potential pressures that may have driven the observed niche partition.
Although we cannot demonstrate the process that has led to the niche partition between the two Stegastes spp., we showed that they are more eco-morphologically different than previously thought. The consideration of large functional groups allows us to detect general changes, divergences between the communities [82–85] but generally fail to capture divergence among closely-related species [11]. Still, the degree of such divergence is essential to understand ecosystem functioning. On reefs, distinct roles have been highlighted within the most common foraging herbivorous such as parrotfishes, surgeonfishes, and rabbitfishes [11]. Similarly, the variation in the role of farming damselfishes needs to be evaluated. In the GC, the two common Stegastes spp. do not share the same ecology which should affect their farming behavior (if such behavior is confirmed for S. flavilatus) and therefore the effect on this so particular behavior on the reef functioning. Our results support the extent to which functional roles may vary between closely related species within a single genus and highlight the importance of validating ecosystem function on a species-by-species basis [14].
Funding
This work was supported by a Marie-Curie BeIPD-COFUND grant (No 600405). Support was received from the SIP-IPN 20172233 project of Instituto Politécnico Nacional to AS.
Acknowledgments
DO was grateful for a post-doc grant from Marie-Curie BeIPD-COFUND. DO is currently a cátedra Conacyt. G.L. is appointed by the National Fund for Scientific Research (FRS-FNRS, Belgium) as a permanent researcher (Research Associate).
Appendix 1 Correlation matrix (Pearson) of the environmental variables
| [1] | [2] | [3] | [4] | [5] | [6] | [7] | [8] | [9] | [10] | |
| [1] | – | – | – | – | – | – | – | – | – | – |
| [2] | 0.04 | – | – | – | – | – | – | – | – | – |
| [3] | 0.03 | 0.03 | – | – | – | – | – | – | – | – |
| [4] | –0.09 | –0.19 | 0.01 | – | – | – | – | – | – | – |
| [5] | 0.02 | 0.04 | –0.03 | 0 | – | – | – | – | – | – |
| [6] | –0.2 | –0.07 | –0.1 | –0.12 | –0.34 | – | – | – | – | – |
| [7] | –0.04 | 0.07 | 0.1 | –0.29 | –0.46 | –0.22 | – | – | – | – |
| [8] | –0.21 | –0.01 | 0.24 | 0.05 | –0.13 | –0.07 | 0.01 | – | – | – |
| [9] | 0.32 | 0.09 | –0.18 | –0.3 | 0.03 | –0.21 | –0.34 | –0.19 | – | – |
| [10] | 0.17 | –0.05 | 0.19 | –0.08 | –0.05 | –0.07 | –0.12 | –0.04 | 0 | – |
Appendix 2 Results of the Generalized Linear Mixed Models on the abundance of Stegastes flavilatus
| Model info | ||||
| Observations | 225 | |||
| Dependent variable | S. flavilatus (individuals/100 m2) | |||
| Type | Mixed effect generalized linear regression | |||
| Error distribution | Negative binomial | |||
| Link function | Log | |||
| Model fit | ||||
| AIC = 474.11 | BIC = 516.61 | |||
| Pseudo-R2 (fixed effects) | 0.06 | |||
| Pseudo-R2 | 0.59 | |||
| Estimates | SE | z-value | P-value | |
| Fixed effects | ||||
| (Intercept) | –1.43 | 0.42 | –3.37 | < 0.001 |
| Depth | 0.13 | 0.32 | 0.39 | 0.69 |
| Rugosity | –0.24 | 0.24 | –1.03 | 0.31 |
| Temperature | 0.33 | 0.36 | 0.92 | 0.36 |
| Sand | –0.21 | 0.24 | –0.87 | 0.38 |
| Filamentous algae | –0.13 | 0.25 | –0.53 | 0.59 |
| Macroalgae | –0.23 | 0.26 | –0.88 | 0.38 |
| Corals | –0.10 | 0.28 | –0.35 | 0.72 |
| Coralline algae | –0.26 | 0.28 | –0.93 | 0.35 |
| Bivalve shells | 0.03 | 0.18 | 0.18 | 0.85 |
| Random effects | ||||
| Group | Std. Dev. | |||
| Sites | 1.45 |
Appendix 3 Results of the Generalized Linear Mixed Models on the abundance of Stegastes rectifraenum
| Model info | ||||
| Observations | 338 | |||
| Dependent variable | S. rectifraenum (individuals/100 m2) | |||
| Type | Mixed effect generalized linear regression | |||
| Error distribution | Negative binomial | |||
| Link function | Log | |||
| Model fit | ||||
| AIC = 2353.39 | BIC = 2399.27 | |||
| Pseudo-R2 (fixed effects) | 0.39 | |||
| Pseudo-R2 | 0.94 | |||
| Estimates | SE | z-value | P-value | |
| Fixed effects | ||||
| (Intercept) | 2.26 | 0.16 | 14.27 | < 0.001 |
| Depth | –0.53 | 0.10 | –5.22 | < 0.001 |
| Rugosity | 0.02 | 0.07 | 0.02 | 0.98 |
| Temperature | 0.18 | 0.13 | 1.32 | 0.19 |
| Sand | –0.49 | 0.08 | –6.20 | < 0.001 |
| Filamentous algae | 0.06 | 0.07 | 0.76 | 0.45 |
| Macroalgae | –0.22 | 0.07 | –3.20 | < 0.001 |
| Corals | 0.02 | 0.09 | 0.29 | 0.77 |
| Coralline algae | 0.07 | 0.08 | 0.88 | 0.38 |
| Bivalve shells | –0.10 | 0.07 | –1.37 | 0.17 |
| Random effects | ||||
| Group | Std. Dev. | |||
| Sites | 0.91 |
Appendix 4 Spatial autocorrelation measured by Moran's I calculated from the residuals from the two Generalized Linear Mixed Models
| GLMMS | Moran's I P-value |
| S. rectifraenum | 0.61 |
| S. flavilatus | 0.93 |
Appendix 5 Food sources contribution in Stegastes flavilatus (from SIAR mixing model). Specimens have been divided in two size categories (according to the median of the standard length). 95% confidence intervals are showed
| Small specimen [68.8–83 mm] n = 11 |
Large specimen]83–95 mm] n = 9 |
|
| Turf | 0–0.23 | 0.014–0.21 |
| Zoobenthos | 0.34–0.70 | 0.38–0.62 |
| Plankton | 0.26–0.49 | 0.32–0.46 |


