1 Introduction
The ecological niche can be defined as an organism's place in its community, its relation to food and enemies, and other factors [1]. The niche has always been closely associated with the assumption that two species cannot coexist if they share a single niche [2]; hence, sympatric species cannot have completely overlapping niches (assuming resources are limiting). In the drive to understand the coexistence of different species and biodiversity in community ecology, the study of trophic niches between sympatric species has been of primary interest [3–5]. Moreover, the successful introduction of non-native species is often explained by the existence of open niches [6].
Recent studies have increasingly considered the ecological and evolutionary significance of individual specialization [7,8]. One level of intrapopulation niche variation widely studied is resource polymorphism, which occurs in a range of vertebrate species [9]. Among fish, resource polymorphism is largely found amongst salmoniforms, however, polymorphism has been recorded from other lineages, including the cypriniforms. Studies on polymorphism in cypriniforms have examined morphological differences between populations [10], whilst others have demonstrated the existence of different morphs within a population [11]. Most studies of fish polymorphism have focused on lacustrine examples, and very few have considered riverine fishes [12,13] and, to our knowledge, no studies have described trophic polymorphism amongst cyprinid fishes. The importance of resource polymorphism is increasingly recognized as a mechanism for resource segregation and the avoidance of competition within population [9,14,15].
The original distribution of the nase (Chondrostoma nasus L., 1758), included central Europe, the Caspian, the Caucasus and the north-west of Asia [16]. The nase was indirectly introduced to the north of France in ca. 1860 via the Rhine. Colonization took place through the canals of western and central France [17]: the establishment of the nase was rapid and it reached the limits of its extension in only 40 years [18]. In southern France, the nase has come into contact with the only other chondrostome present in the French freshwater ichtyfauna, Chondrostoma toxostoma (Vallot, 1837), (the sofie). The sofie is smaller and endemic to southern France. In the Durance basin, contact between the two chondrostome fishes results in hybridisation [19]. The rapid colonisation of French catchments by nase is associated with their specialised diet [16]. The nase has been described as a benthic herbivore, consuming epilithic algae, particularly diatoms [16,20]. Little is known regarding the diet of sofie, apart from a study by Chappaz et al. [21] who revealed that in a reservoir located within the Durance catchment, sofie had a diverse diet that included algae and aquatic invertebrates. There have been no studies examining the diet of Chondrostoma Spp. hybrids.
Previous studies have used stomach contents analysis to examine the trophic ecology of chondrostome fishes. However, this method is somewhat limited, as it only provides a snapshot of recently ingested food items (rather than assimilated diet), and in fishes that macerate their prey (like chondrostomes) it can be difficult to reliably identify and estimate prey consumption. An alternative and powerful approach to the study of trophic ecology is the measure of stable isotopes [22–24]. Moreover, nitrogen (δ15N) and carbon (δ13C) stable isotopes permit the definition of both trophic and spatial niches of a species [25,26], because an animal's chemical composition is directly influenced by what it consumes, as well as the habitat in which it lives. The use of stable isotope data has recently shifted from a qualitative to quantitative approach to describing food webs, due to the generation of a series of different metrics [27,28]. Here, we use these metrics to examine diet overlap between a species-pair.
Due to the phenomenon of introgression, the Chondrostoma species-pair provided a good model to study resource partitioning. Costedoat et al. [19] reported differences in chondrostome abundance between different sections of the River Durance separated by dams. The most upstream section of the Durance basin (the Buech River) supported a high relative abundance of all three chondrostomes, i.e., nase, sofie and their hybrids. The aim of the current study was to examine resource partitioning at both inter- and intraspecific levels to improve our understanding of the coexistence of fish species using the case of the chondrostome species-pair in the Buech River. Using stable isotope analysis, we studied chondrostome trophic and spatial niches by: (1) evaluating chondrostome migration through the isotopic characterisation of different feeding habitats, (2) taking into account the influence of biological variation on stable isotope values, (3) quantitatively analysing trophic niche overlap and (4) examining variation in life history traits between the chondrostome species-pair (gonadosomatic ratios (GSR), maturity, growth and morphology). Finally, we discuss the effect of individual specialisation at the level of population and the chondrostome species-complex.
2 Materials and methods
2.1 Study site
The study focuses on the Durance basin, a tributary of the Rhone River – the principal river of the south of France (Fig. 1). A characteristic of this basin is the presence of several dams, which prevent fish movement, due to a lack of fish passes. The study area was located at the confluence between the Durance and Buech Rivers (Fig. 1). Dams are present at the confluence, in the Buech River ca. 35 km upstream of the confluence, and in the Durance river ca. 25 km upstream of the confluence (Fig. 1). Although regulated by a dam, the Buech River retains its torrential characteristics, with a minimum discharge of around 0.9 m3 s−1, and a mean width of ∼6 m and depth of 0.8 m. The Durance River has a minimum discharge of ca. 3 m3 s−1 with a mean width of ∼8 m and depth of 1 m. The lowest section of the Buech River located ca. 500 m above the confluence receives canal water discharge originating largely from the Durance basin (Fig. 1). From this point, the features of the river change completely: the mean width and depth of this section are ∼30 m and 2 m respectively, although these fluctuate seasonally. Mean discharge in this section is ca. 250 m3 s−1, ranging between 80 and 400 m3 s−1 over the year.

Location of the Durance River and Buech River including dams, and the position of invertebrate and fish sampling sites.
2.2 Collection of fish and invertebrate samples
Fish were collected by electrofishing of spawning habitats in the Buech River during the 2001 (April–May) spawning season (Héron, Dream Electronic Equipment, Generator, DC, 300 V, 3A and one anode). The Buech River supports higher chondrostome densities than the Durance River [29]. Surveys were conducted over four different days between April and May 2001. Several sites were sampled in the Buech River, at ca. 14 km upstream to the confluence (Fig. 1). In a ca. 4 km stretch, 4 sites were sampled, characterised by the presence of a succession of riffle and pools, which correspond to chondrostome spawning habitats.
First, to examine the influence of spatial origin on nase isotope variability, we conducted spatially resolved sampling of invertebrates from both the Buech and Durance Rivers (Fig. 1). Macroinvertebrates were collected on riffles, using surber net (500 μm), at 5 locations along a ca. 18 km stretch of the Buech River (Fig. 1: no. 1 to 5). A final station (no. 6) was sampled in the lower, modified section of the Buech River. Two stations (no. 7 and 8), separated by ca. 1 km, were sampled on the Durance River.
Next, the relative position of each chondrostome isotope value was deduced from visual comparison with those of primary consumers. Three families were analysed separately: Baetidae, Oligoneuriidae (Ephemeroptera) and Hydropsychidae (Trichoptera). The sampling was conducted on riffles with a standard kick net (mesh 500 μm). Twenty-two samples were made by station and by family; only Oligoneuriidae in stations 6 and 8 were not made, due to a lack of individuals for δ13C and δ15N analyses. These families were chosen for their high abundance, their presence in the different stations and the role of Baetidae as primary consumers.
2.3 Biological variables
Chondrostome fishes in the area surveyed spawn between March and the end of May (unpublished data). Due to the issue of introgression between nase and sofie, the taxonomic status of each fish was examined using molecular methods [30]. Using data from Costedoat et al. [30], fish were classified as: nase, sofie or nase-sofie hybrids. For each fish, fork length (F, in cm) and mass (W, g) were recorded and scales removed for age and growth determination. Both growth and reproductive variables were studied: growth variables included LF, W and the annual growth increment. Only the last annual increment was considered and inferred from scalimetry (log F = log u + v log SL, with F: fork length and SL: scale length [31]). The reproductive variables measured included sex, maturity status and the gonadosomatic ratio (GSR). Sex was composed of three classes: female, male and not attributed. Maturity data included two classifications: mature or immature and was defined using GSR.
2.4 Morphometric variables
Fish were photographed with a Nikon Coolpix 995 camera and shape characterized by landmarks, using TPSdig software. Thirteen homologous landmarks were recorded to describe fish shape (Fig. 2) [30]. Landmark coordinates were transformed to define shapes which were projected into a tangent shape space to allow multivariate analysis [32]. Those morphological variables would be used to compare multiple groups of fish with different size compositions which might induce confounding differences between groups due to allometry [33]. In order to remove allometric effects on shape, residuals of the multivariate linear regression of tangent coordinates on individual size were used [34]. Those residuals were considered as the morphological variables for the rest of the study.

a. Location of landmarks (n = 13) used to characterise fish shape (1: dorsal breakdown due to dorsal fin–2: anterior insertion of dorsal fin–3: posterior insertion of dorsal fin–4: intermediate point of caudal fin insertion–5: ventral insertion of caudal fin–6: anal fin insertion–7: pelvic fin insertion–8: pectoral fin insertion–9: ventral limit of head–10: ventral limit of preopercule–11: anterior extremity–12: dorsal limit of head–13: eye). b. Thin-plate spline transformation grids from nase group 1 mean shape to nase group 2 mean shape. Masquer
a. Location of landmarks (n = 13) used to characterise fish shape (1: dorsal breakdown due to dorsal fin–2: anterior insertion of dorsal fin–3: posterior insertion of dorsal fin–4: intermediate point of caudal fin insertion–5: ventral insertion of caudal fin–6: anal ... Lire la suite
2.5 Stable isotope analyses
Fish stable isotope ratios (δ13C and δ15N) were measured from dorsal muscle tissue. Invertebrate stable isotope values were estimated from composite samples: values for Baetidae were estimated from ca. 25 individuals, both Oligoneuriidae and Hydropsychidae values were estimated from ca. 4 individuals. All samples were dried at 60 °C for 24–48 h (according to the protocol of the Colorado plateau stable isotope laboratory), ground with a mortar and pestle and weighed into tin-capsules prior to combustion in an elemental analyser (ECS4010, Costech Analytical Technologies, Inc.) coupled to an isotope ratio mass spectrometer (Thermo-Finnigan Deltaplus Advantage, Thermo Scientific). Carbon and nitrogen stable isotope values are expressed in conventional delta (δ) notation, defined as per mille (‰) deviation from an international standard, which is Pee Dee Belemnite limestone (PDB) for 13C/12C and atmospheric N2 for 15N/14N, with standard deviation of 0.3‰ for δ15N and 0.1‰ for δ13C. δ13C values of untreated and lipid-extracted samples were compared for a subset of individuals, but showed no evidence of any effect of lipids on δ13C values. As such, δ13C data shown here reflect raw δ13C values.
2.6 Statistical analyses
Variation in macroinvertebrate isotope values between the Buech River (stations 1 to 5 in Fig. 1) and the lower Buech and the Durance Rivers (stations 6 to 8) was examined through a Mann-Whitney test. Using stable isotope values, the chondrostome fishes were classified into 2 groups (by comparisons with macroinvertebrate values) using a k-means clustering approach [35].
No statistical analyses were conducted with fish from Group 2 due to the low sample size. The effects of biological variation on stable isotope values were removed from fishes from Group 1 using an analysis of covariance (Fig. 3a). The ANCOVA was performed separately on carbon and nitrogen values for each taxon (nase, sofie & hybrids). The model included all categorical variables (sex, maturity and age) and their first order interaction. Even if categorical variables were not significant, they remained in the model to control for all biological effects. The purpose was not to fit the best regression but to account for the effect of all factors. Quantitative variables (size, last growth increment and GSR) were added to the model as covariates if they were significantly correlated with the stable isotope values. Therefore, isotopic variables corrected from biological influence could be constructed by adding ANCOVA residuals to the biological adjusted mean.

Variation in stable isotope values including (a) scatterplot showing δ13C and δ15N of nase (■ group 1, □ group 2), sofie (• group 1, ○ group 2) and hybrids (▴ group 1, Δ group 2) and (b) their trophic niche in the Buech river as represented by the convex hull of δ13C-δ15N space.
Isotopic niche overlap was examined in each of the river systems based on the size-corrected isotopic variables. Niche width was defined by the dispersion of the stable isotope values and represented via convex hulls [27]. The total community niche hypervolume reflected the total isotopic space inhabited by the 3 chondrostome taxa. The total community hypervolume was divided into 3 categories [36]: (1) the secondary level of intersection, represented by the niche hypervolume shared by all 3 taxa; (2) the primary level, represented by the niche hypervolume shared by 2 taxa; and (3) the hypervolume utilised by a single taxon.
Trophic polymorphism was examined with regard to the two subpopulations with different spatial origins. Comparisons were made on morphological and biological variables. As sofie sample sizes were small, they were discounted from the analysis. Each biological variable (size, last increment, GSR) was examined using ANOVA including age, sex, maturity, subpopulation origin, and first order interactions [37]. Normality of the residuals was confirmed by visual inspection (quantile plots) and via Shapiro tests. Morphological variables were transformed into principal components (PCA) before using a MANOVA test to compare for differences between taxa [37]. The same linear model as described for the biological variables was used. Significant morphological differences between subpopulations were visualised using a deformation [32]. All statistical analyses were conducted within the R statistical environment – v2.3.1 (2008), using an alpha value of 5%.
3 Results
3.1 Data set
One hundred and seventy-six individual chondrostomes (77 nase, 60 sofie and 39 hybrids) were caught and examined. Nase fork length varied between 93 and 315 mm; sofie between 87 and 192 mm and hybrids between 90 and 288 mm (Table 1). The majority were aged either 2 (30%) 3 (33%) or 4 (37%) years old.
Variation in key biological characteristics (Mean ± SD) between nase, hybrids and sofie classified by cluster analysis as either Group 1 or 2 (G1 and G2, respectively). Also shown is the abundance of individual chondrostomes classified in each group by age-class.
| n | δ13C (‰) | δ15N (‰) | Length (mm) | Final growth increment (mm y−1) | Mass (g) | GSR | Contribution to survey catch by age-class | |||
| 2 years | 3 years | 4 years | ||||||||
| Nase G1 | 56 | −28.0 ± 2.4 | 7.5 ± 1.6 | 199 ± 60 | 59 ± 21 | 115.7 ± 98.8 | 1.2 ± 2.0 | 17 | 16 | 23 |
| Nase G2 | 21 | −24.4 ± 2.1 | 4.9 ± 1.4 | 184 ± 69 | 61 ± 19 | 82.1 ± 99.7 | 1.1 ± 0.9 | 12 | 6 | 3 |
| Hybrid G1 | 25 | −27.3 ± 2.4 | 7.7 ± 1.7 | 182 ± 49 | 48 ± 19 | 81.5 ± 67.7 | 1.3 ± 7.7 | 6 | 9 | 10 |
| Hybrid G2 | 14 | −24.5 ± 1.6 | 5.3 ± 1.1 | 217 ± 50 | 57 ± 28 | 123.0 ± 98.5 | 8.2 ± 2.6 | 2 | 6 | 6 |
| Sofie G1 | 52 | −27.7 ± 2.5 | 7.8 ± 1.0 | 144 ± 25 | 35 ± 12 | 35.2 ± 15.4 | 6.9 ± 4.6 | 14 | 17 | 21 |
| Sofie G2 | 8 | −24.3 ± 1.9 | 5.9 ± 1.0 | 147 ± 29 | 35 ± 15 | 34.7 ± 20.4 | 6.4 ± 9.8 | 2 | 4 | 2 |
3.2 Spatial isotope variability
Invertebrate mean (± SD) δ13C and δ15N values were −30.7 ± 2.9‰ and 6.8 ± 1.6‰, respectively. The large amount of isotopic variation reflected a difference between the Buech River (no. 1 to 5) and both the lower Buech and the Durance Rivers (no. 6 and, 7 and 8) (Mann Whitney: P < 0.001). Invertebrate δ15N values (mean ± SD = 7.7 ± 1.0‰) from the Buech River were enriched relative to those from the lower Buech and Durance Rivers (4.8 ± 0.8‰). Conversely, invertebrate δ13C values in the Buech River were depleted (−32.2 ± 1.8‰) compared to those collected from the lower Buech and Durance Rivers (−27.0 ± 0.9‰: Fig. 4).
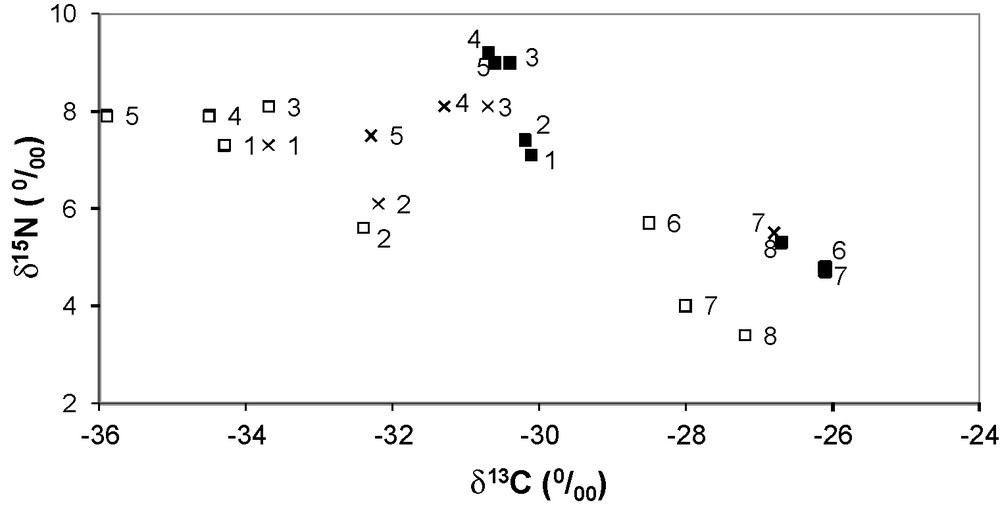
Stable isotope biplot showing variation in δ13C and δ15N of different invertebrate families (Hydropsychidae ■, Baetidae □ and Oligoneuriidae x) sampled from 8 distinct sites in Buech River and Durance River (Fig. 1).
A cluster analysis allowed the chondrostomes to be classified into two groups based on δ15N values (Fig. 3a), but they also differed in terms of δ13C values (Table 1). Individuals in Group 1 were relatively enriched in 15N and depleted in 13C than their equivalents in Group 2, and due to the similarity to the differences in macroinvertebrate isotope values between the two systems, we suggest that fish from Group 1 were resident in the Buech River, whilst Group 2 originated from the lower Buech and Durance Rivers. The relative contribution of the different taxa to the two groups is shown in Table 1.
3.3 Effects of biological variation on fish stable isotope values
Analyses were performed only on fishes from Group 1 due to the limited sample from Group 2. Sample size requirement for the covariance analysis was not fulfilled for the first order interaction of maturity with age and also, for sofie, with sex. Hence, these effects could not be included in the models. Generally, stable isotope variability in nase appeared to be more associated with biological variables than the other chondrostomes (Table 2).
Biological variables significantly correlated with Group 1 fish δ13C and δ15N values.
| Significant correlation | ||
| δ15N | δ13C | |
| Nase | Size (r = 0.30, P = 0.02) | Size (r = 0.50, P = 8 × 10−5) Increment (r = 0.34, P = 0.009) GSR (r = 0.33, P = 0.01) |
| Sofie | Increment (r = 0.28, P = 0.04) | |
| Hybrids | Increment (r = −0.41, P = 0.04) | Size (r = 0.45, P = 0.02) Increment (r = 0.50, P = 0.01) |
The full model (all categorical variables) with the correlated variable as a covariate (Table 2) was selected to best remove the maximum biological influence on isotope values. One categorical variable, maturity, was significant (ANCOVA, F = 4.2, df = 1,38, P = 0.048) and possibly reflects variation in carbon values in the nase.
3.4 Trophic niche overlap
Comparison of trophic niche overlap was restricted to Group 1 individuals due to their supposed similar spatial origin. The secondary level of intersection (i.e., the niche hypervolume shared by all 3 taxa) in isotopic niche space was largely higher than both the primary level of intersection and the niche hypervolume with one species (Table 3 and Fig. 3b). The niche hypervolume of the three chondrostomes was quite similar.
Overlap of the isotopic niche space between chondrostome taxa, expressed as percentages of the total community hypervolume, and niche hypervolumes for sofie, nase and hybrids.
| % of community | |
| Secondary level of intersection | 53.4 |
| Primary level of intersection | 74.3 (53.4 + 20.9) |
| Hypervolume with one species | 25.7 (100 – 74.3) |
| Sofie and nase | 63.5 |
| Sofie and hybrids | 60.2 |
| Nase and hybrids | 57.5 |
3.5 Polymorphism
No differences in life history traits were observed in hybrids from the two different groups. Nase length differed between the two groups (simple effect of groups: F = 6.18, df = 1,52 P = 0.02, no significant interactions with groups at a 5% level). Fish from Group 2 were on average ca. 20 mm larger (i.e., ca. 10% of the average size of the nase sampled) than fish from Group 1. Furthermore, mean differences in morphology were observed between the two groups (simple effect of groups: MANOVA; F = 1182.14, df = 22,38, P < 0.001, no significant interactions with groups at a 5% level). Typically, fishes from Group 2 appeared thinner in the ventral part with an extended caudal region (Fig. 2). MANOVA group coefficients only showed a strong association with the first axis of the morphological PCA (Partial R2 ca. 90%). This first axis explained 74% of shape variability. Therefore, group membership explained about 66% (90% of 74%) of shape variability. Finally, ANCOVA of nase carbon and nitrogen isotope values showed no significant interactions between biological factors or covariates and groups.
4 Discussion
4.1 Spatial origin
Invertebrate stable isotope values showed a marked spatial pattern, where samples from the Buech River were clearly distinguished from those from the lower Buech and Durance Rivers. Notably, δ15N values were strongly divergent between the two different areas. The two fish groups were distinguished mainly by nitrogen (Fig. 3a), and the differences in nitrogen were similar to that shown by invertebrates from the two different spatial origins. However, the fish also clustered in terms of their δ13C values; the chondrostomes included some individuals that were more 13C depleted and some that were more 13C enriched than the invertebrates. Variability in δ13C could be explained by both sampling bias and the high δ13C variability in Durance basin invertebrates. Only riffle habitats were sampled and Finlay et al. [38] observed δ13C differences between riffle and pool habitats, where pool habitats were typically 13C enriched. Likewise, the chosen families were not fully representative of the river food web. Other results from a lower section of the Durance River showed significant seasonal variation in Hydropsychidae δ13C values [39]. Conversely, in a downstream part of the Durance River, we reported little seasonal variability in Hydropsychidae δ15N values between the main river and a tributary [39]. This result strengthens the existence of two isotopically-distinct habitats in the survey area. Within the Buech River, the lower sections showed similar macroinvertebrate δ15N values to those reported from the Durance River. This likely reflects the isotopic influence of large volumes of canal water, which largely originates from the Durance basin. Following Hansson et al. [40] and Harrington et al. [41], the similarity between the spatial patterns displayed by macroinvertebrate δ15N values and the variability in chondrostome δ15N indicates that although captured from one area, the chondrostomes actually originated from two different sources.
Alternative hypotheses to explain the variation in chondrostome δ15N values could be differences in diet or physiological processes [22,42,43]. Nase displayed greater variation in δ15N than the other chondrostome taxa (Fig. 3a and Table 1). However, studies of nase diet from both Switzerland and the Rhone River (France) highlighted the dominance of algae in the diet. Diatoms dominated gut contents and animal prey was rarely consumed. As such, nase have been strictly considered as primary consumers and trophic specialists [18,20]. In the two groups of nase identified here, we found no evidence of differences in the relationship between various biological variables and δ13C and δ15N. This indicates that the influence of various physiological processes (growth, reproduction, etc.) on isotopic values appear to be similar for both groups.
In addition to stable isotope values [44], morphology can be used as a marker of membership of ecologically distinct groups [35]. The differences in morphology we demonstrated between individuals with different isotopic values strengthen the hypothesis of different spatial origins. Although we did not show morphological differences between the hybrids from Group 1 and Group 2, (probably due to the hybridisation phenomenon [30] or the low sample size), and our inability to compare sofie groups due to low sample size in Group 2, we feel that both sofie and hybrids showed a similar behaviour compared to nase. We suggest that spatial variability had a greater role in driving the isotopic variability found in the chondrostome fishes in our study-area than physiological processes and diet. Furthermore, we suggest that the isotopic variation in the chondrostomes reflects evidence for spawning migration, where individuals from Group 1 originated from the Buech River, whilst individuals from Group 2 originated from either the lower Buech River or the Durance River.
Nase dominated those fish that we suggest had migrated from the lower Buech or the Durance Rivers. Nase have been classified as in decline in many rivers [45,46]. Zbinden et al. [46] and Ovidio and Philippart [47] suggested that habitat fragmentation was the main cause of nase decline, driving a realization that habitat connectivity should be maintained to permit the continuation of the nase life cycle. Several authors [48,49] have shown through mark-recapture that nase movements can vary between tens of kilometres to more than 100 km. Stable isotopes represent a less time-consuming, and less expensive means to track migration and also permits the behaviour of individuals that are too small to be tagged to be studied [25]. Stable isotope techniques are increasingly used to infer the spatial origin of organisms at large scales (e.g., continental [50,51]). Our data contribute to the rather limited number of studies conducted either at a smaller scale (i.e., a few kilometres, [44,52]) and especially on fish, according to [42]. Another interesting result from this study reflects the behaviour of hybrids. Hybrid chondrostomes were more abundant in Group 2 (36%) than either parental species. This raises the question of the status of the hybrid niche, relative to the two parental species.
4.2 Trophic niche overlap
If, as we conclude, that fish from Group 1 originated from the Buech River, there was no evidence of trophic resource partitioning between the sympatric species-pair (nase v sofie) found in this river. Other authors have observed the coexistence of closely related species without any apparent partitioning of food resources [53,54]. The δ13C-δ15N niche space of the three chondrostome fishes from the Buech River (i.e., Group 1) showed considerable overlap; overlap between two chondrostome taxa was around 60% and little niche space occupied by only one chondrostome taxon (25.7%, details in Table 3). Given the elevated abundance of the three chondrostome fishes in the study area [19], the scope for ecological interactions is potentially higher relative to that from other areas of the Durance basin. Stable isotope results from the other sections (unpublished data) supported our results including the overlap in δ13C-δ15N isotope niche space. The establishment of the nase in the Durance basin probably did not reflect the invasive nase inhabiting a vacant trophic niche previously unexploited by the endemic sofie. It also appears unlikely that the coexistence of the hybrids with the parental species is driven by different trophic resources.
However, stable isotope values reflect the long-term assimilated diet of consumers, the time period that they reflect is influenced by tissue turnover rates [55]. In spite of the difficulties involved in estimating tissue turnover times [56], Perga and Gerdeaux [55] suggested that muscle tissue in temperate freshwater fishes may relate to a period of ca. 6 months. So, in our case, stable isotope values mainly reflected the proceeding winter period. In a different period, the overlap between chondrostomes can be lower as observed by diet studies on the two parental species [16,20,21]. The influence of different biological factors (age, growth, etc.) which affect the assimilation of food-derived energy and nutrients into body tissues [57], on stable isotope values was not consistent between the chondrostomes studied here. Although we feel that such confounding factors played little role here, as isotopic overlap was so high, their influence should be considered carefully in future stable isotope studies.
4.3 Resource polymorphism
We examined ecological polymorphism within chondrostome subpopulations originating from two putative spatial areas. As all individuals were captured from one spawning habitat in the Buech River, we were unable to perform an a priori classification as in [44]. As our a posteriori classification was not based on clear classification rules, then individuals located at the boundary of the two groups may have been misclassified. However, our results indicated that nase displayed resource polymorphism. The two nase morphs showed differences in morphology, behaviour (migrant and resident individuals) and performance (growth rate). Origin accounted for 66% of nase morphological variability, where individuals that we have identified as originating from the lower Buech or Durance Rivers showed an increase in both postanal (landmarks 4 and 5) and predorsal (landmarks 2 and 3) distances (Fig. 2). These shape differences and an increased mean length both indicate an increased size of the lateral surface area. These differences are likely to be associated with improved swimming performance [58]. Hence, the individuals that migrated from the lower Buech or Durance Rivers in order to spawn in the Buech River, showed morphology more suited to their home habitat. Although resource polymorphism is widely studied in fishes (review by [9]), the majority of studies have concerned lake populations. To our knowledge, this is the first study showing polymorphism in riverine cyprinids.
5 Conclusion
Resource partitioning represents a fundamental concept in community ecology, and classical competition theory predicts that when resources are limited, sympatric species cannot have completely overlapping niches. However, this hypothesis was not validated in our study of chondrostome fishes. Resource polymorphism is recognised as a means permitting closely related taxa to partition resources, and hence avoids competition [9,14]. Intraspecific competition for food is typically recognised as the mechanism driving resource polymorphism [15,59]. In the case of the chondrostome fishes described here, it appears that given the high overlap in food niches, more than the intraspecific competition, interspecific competition can promote individual variation. Consequently, resource polymorphism was likely an outcome of the coexistence of the chondrostome pair species. This study was an example of the link between individual variation and coexistence of different pair species. Differences between morphs were not explained by age or sex. As the nase polymorphism was apparent as early as 2 years, it is likely that morphological differences exist between young of the year individuals, as observed in anadromous and resident salmonids [13]. As observed by Darimont et al. [60] and Swanson et al. [61], it is likely that the ecological factors maintaining the nase polymorphism is spatial heterogeneity, and future work should examine whether the polymorphism has a genetic basis.
6 Conflict of interest statement
No such conflicts exists.
Acknowledgements
This work was supported partly by a grant from “region Provence Alpes Côte d’Azur”. We are grateful to the officers of the “Office national de l’eau et des milieux aquatiques” who helped us with each sample and showed great interest in the study. We would like to thank Costedoat C. and Gilles A. for the access to the genetic determination and morphometry of the chondrostomes. The Max Planck Society funded J. Grey and C. Harrod. Thank you to an anonymous reviewer for his comments on this manuscript.


