1. Massaging the oocyte nucleus for a good quantitative and qualitative transcriptome
1.1. A non-specific gradient of pressure positions large objects in the centre of the oocyte
The position of the nucleus in a cell can instruct morphogenesis in some cases, conveying spatial and temporal information. Abnormal nuclear positioning can lead to disease [1]. In oocytes from many species, nucleus position regulates embryo development. For example, in Drosophila it is located under the antero-dorsal oocyte cortex and defines the future dorso-ventral axis of the embryo and of the adult body plan. However, in mammals, the oocyte nucleus is centrally located and does not instruct any future embryo axis. Yet an off-centre nucleus correlates with a poor outcome for mouse and human oocyte development [2, 3]. This is surprising since oocytes further undergo two extremely asymmetric divisions in terms of the size of the daughter cells (enabling polar body extrusion), requiring off-centring of their chromosomes (Figure 1).
Scheme of the end of oocyte growth, oocyte divisions and early development after fertilization. The nucleus (pink) is centred at the end of oocyte growth thanks to the presence of increasing acto-myosin-based forces. Oocytes are arrested in prophase I in the ovary and will resume meiosis upon hormonal trigger. Meiosis resumption is marked by nuclear envelope breakdown (NEBD). The first meiotic spindle assembles in the absence of canonical centrosomes. It migrates towards the closest cortex, which permits a first asymmetric division in size (extrusion of the first polar body, 1st PB), allowing the oocytes to preserve the maternal inheritance accumulated during growth. There is no DNA replication between the two meiotic divisions, which permits the formation of a haploid gamete after second polar body extrusion (2nd PB). Fertilization occurs after ovulation, in the oviduct, when the oocyte is arrested in metaphase of the second meiotic division (Metaphase II) and at that stage the second meiotic spindle stays anchored into the cortex. The arrow represents chromosome motion.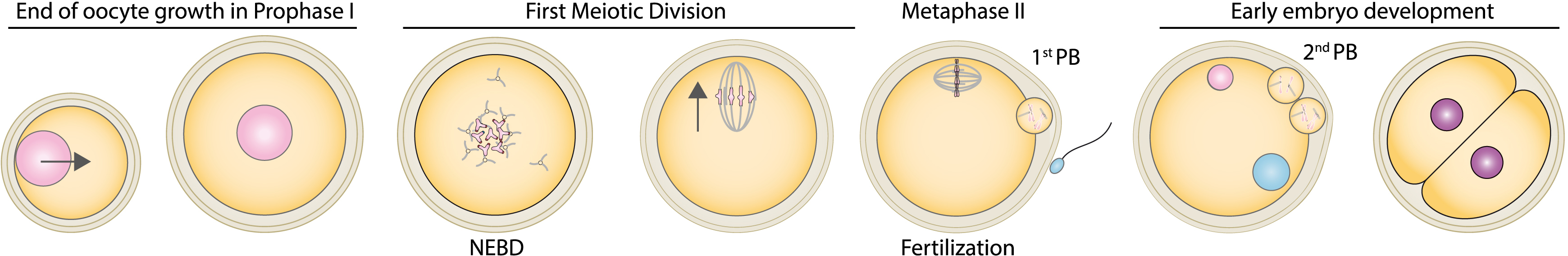
We discovered that in the mouse, the nucleus is positioned at the centre of the oocyte via an acto-myosin gradient of pressure [4]. This gradient of pressure arises from a gradient of velocities of actin-positive vesicles. These vesicles are positive for Rab11a [5] and two essential actin nucleators, Spire 1 & 2 as well as Formin 2 are anchored onto their membrane [6, 7]. As a consequence, actin filaments grow from the vesicles and this is why we name them actin-positive vesicles. Furthermore, these vesicles are cargos for Myosin Vb [5, 8] and we showed that when the acto-myosin Vb meshwork is dynamic (growing and shrinking and containing vesicles that move along the actin filaments via the Myosin Vb motor activity), it propels the whole cytoplasm by advection (Figure 2). We demonstrated, using biophysical and 3D agent-based simulations that this gradient is non-specific and propels large objects towards the oocyte centre both during prophase I and meiosis I, allowing the oocyte to preserve its large organelle content, despite undergoing asymmetric divisions [9] (Figure 3).
Scheme representing the acto-myosin based forces which promote nucleus centering. (A) Global scheme of F-actin (red filaments) and Myosin Vb motor (violet) anchored into Rab11a-positive vesicles (red circles) as shown in [4, 5, 6, 7, 8]. (B) Zoom on the effect of Rab11a-positive vesicle motion on the cytoplasmic fluid, moving by advection.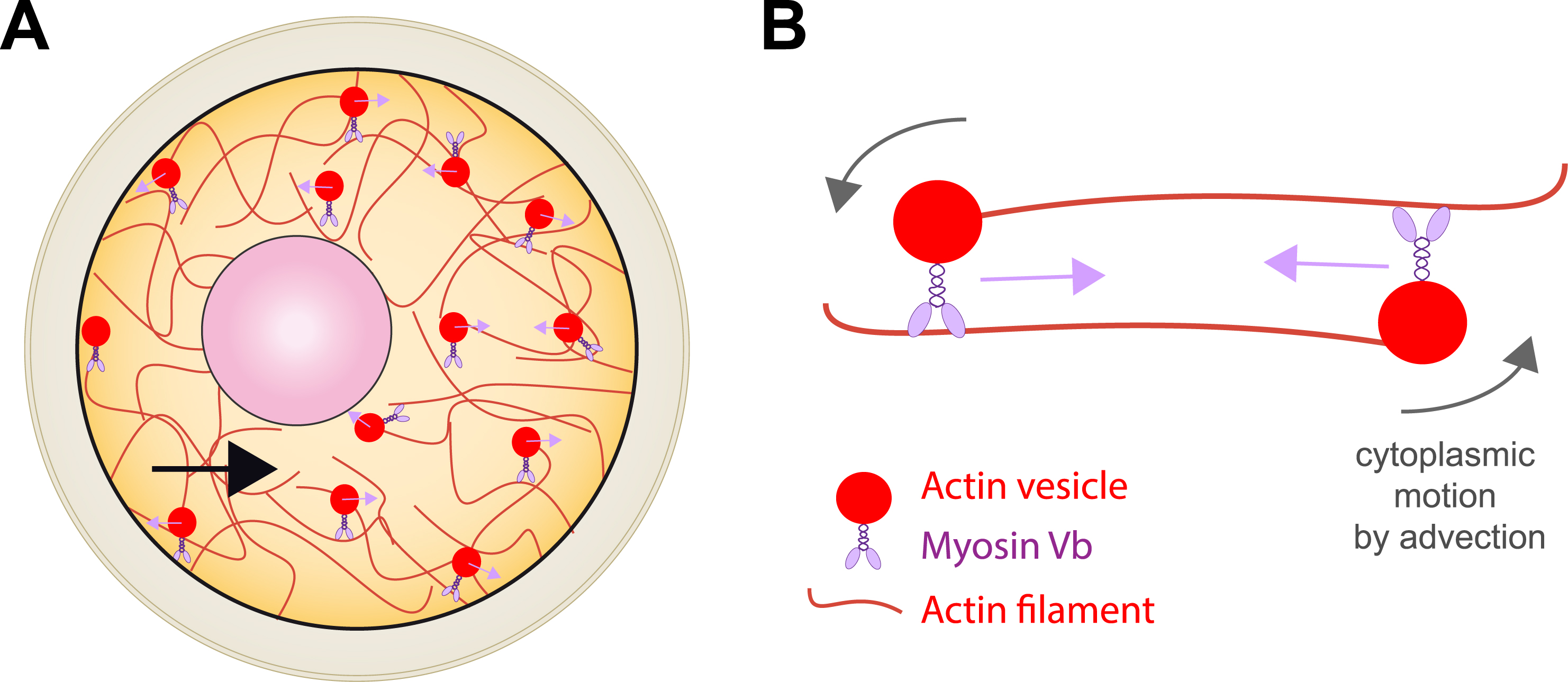
Confronting simulation to experiments to validate the non-specific nature of the gradient of pressure. Left: Oocytes arrested in prophase I and microinjected with inert objects, such as oil droplets (white arrows). These oil droplets mimic the nucleus behavior, in terms of F-actin recruitment (right picture) and capacity to become centralized with velocities comparable to the nuclear ones. Right: Modelling and 3D-simulations of the motion of inert objects subjected to the gradient of pressure of actin-positive vesicles allow to confront theory and experiments [9]. Scale bar: 10 μm.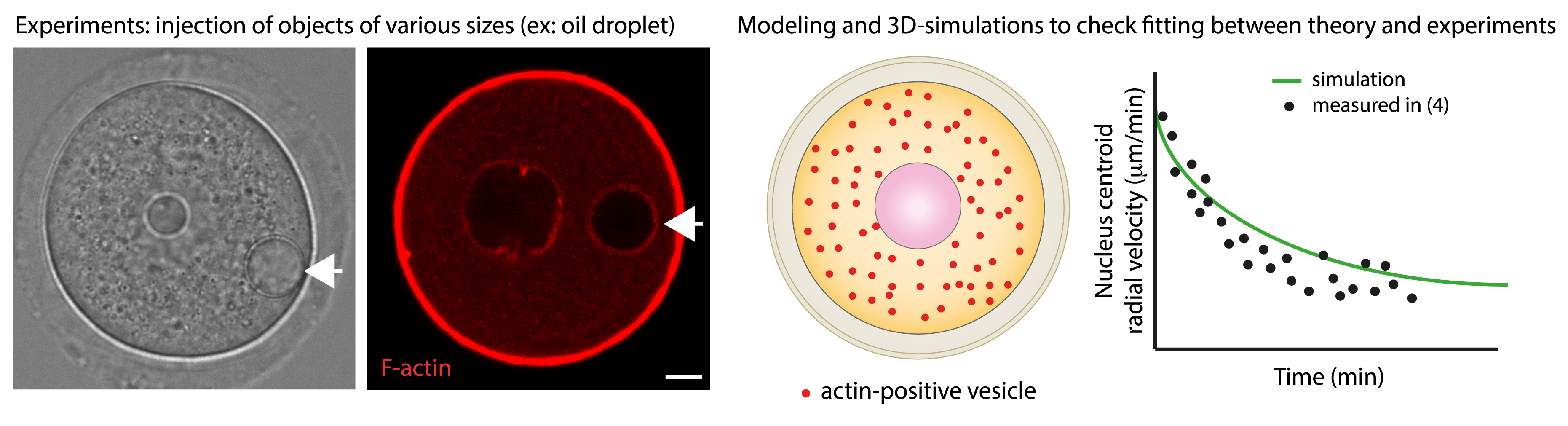
1.2. A mechano-transduction cascade controls gene expression in the oocyte
Importantly, we showed that forces from the acto-myosin cytoskeleton, which position the nucleus, increase in intensity during the last stages of oocyte growth in the ovary, due to an increase in F-actin density [10]. As a consequence, advection of the cytoplasm rises during these last stages of growth and more forces impact the nuclear envelope. We quantified the fluctuations of the nuclear envelope and showed that they depend on the presence of F-actin [11, 12]. The oocyte nuclear envelope undergoes prominent and important shape changes, arguing that it is massively deformable and not extremely rigid. By performing RNA sequencing comparing control versus oocytes that are devoid of a key oocyte cytoplasmic F-actin nucleator, Formin 2 [13, 14, 15], we observed a significant downregulation of about 200 genes in oocytes devoid of acto-myosin based forces from the cytoplasm [11]. We also observed a reduction in the motion of chromosomes inside the nucleus in those mutant oocytes [11]. Since these genes are distributed randomly in the mouse genome, we believe that a mechano-transduction cascade agitates the whole nucleoplasm, promoting the transcription of genes that are active at that stage.
1.3. A mechano-transduction cascade controls splicing activity in the oocyte
In addition, we showed that these nuclear fluctuations also affect nuclear organization, in particular nuclear body organization. Nuclear bodies are extremely dynamic membrane-less organelles which are essential accelerators of mRNA processing. We demonstrated that growing mouse oocytes experience an increase in cytoplasmic acto-myosin based forces that boost nuclear condensate fusion (Figure 4) [10]. When biomolecular condensates increase their mobility, they have a greater chance of colliding with each other and because they are liquid-like, the chances of fusion also increase. This leads to a decrease in the number of biomolecular condensates and to an increase in their size, as we observed in oocytes [10] (Figure 4). Nuclear bodies are distinct membrane-less organelles which play essential roles in the control of gene expression. Among those best characterized so far, one can find the nucleolus, which has essential functions in ribosomal subunit biogenesis; Cajal bodies, which participate in the maturation of small nuclear and small nucleolar ribonucleoprotein particles (snRNPs and snoRNPs); nuclear speckles, essential for transcription and mRNA maturation and processing; histone locus bodies, associated with the biogenesis of histone mRNAs; PML (ProMyeloid Leukemia) bodies, involved in the cell cycle and genome repair and maintenance; and paraspeckles, which regulate gene expression through sequestration of specific transcripts and protein components [16, 17, 18]. These nuclear bodies, also named nuclear condensates, are composed of specific RNAs and proteins and can be recognized using specific markers. Importantly, these condensates cooperate globally inside the nucleus to transcribe, process and export RNAs towards the cytoplasm, accomplishing key functions in the cell. Nuclear speckles extensively contribute to various RNA maturation and splicing [19]. The capacity of the cytoskeleton forces to organize the nucleoplasm, and in particular nuclear speckles, has been conserved during the evolution of species being also observed in growing Drosophila oocytes [10]. We also showed that larger nuclear speckles display increased splicing activity, suggesting the presence of a cascade of energy transfer from the acto-myosin mechanical forces towards motion of nuclear bodies and up to molecules inside the nuclear speckles (Figure 4). This energy transfer enhanced condensate-associated activity such as active splicing, modulating mRNA processing [10] (Figure 4). Beyond reproduction, this mechanism sheds new light on numerous anomalies linked to defects in cytoplasmic forces correlating with defects in condensate architecture observed in many cancers [20, 21], as is the case of PML nuclear bodies in Acute Promyeloid Leukemia (APL-type of Leukemia) [22].
Scale-crossing activity of forces from the cytoskeleton inside mouse oocytes. Left: picture of a fully-gown oocytes observed by transmitted light, where chromosomes appear in pink. Scale bar: 10 μm. Right: scheme representing how increasing acto-myosin based forces promote nucleus motion, nuclear envelope fluctuations, nuclear bodies coarsening and increase of molecular mobility and hence splicing activity inside nuclear speckles. F-actin in red, nucleus in pink, nuclear bodies in various colors, nuclear speckles in violet and molecules in green circles.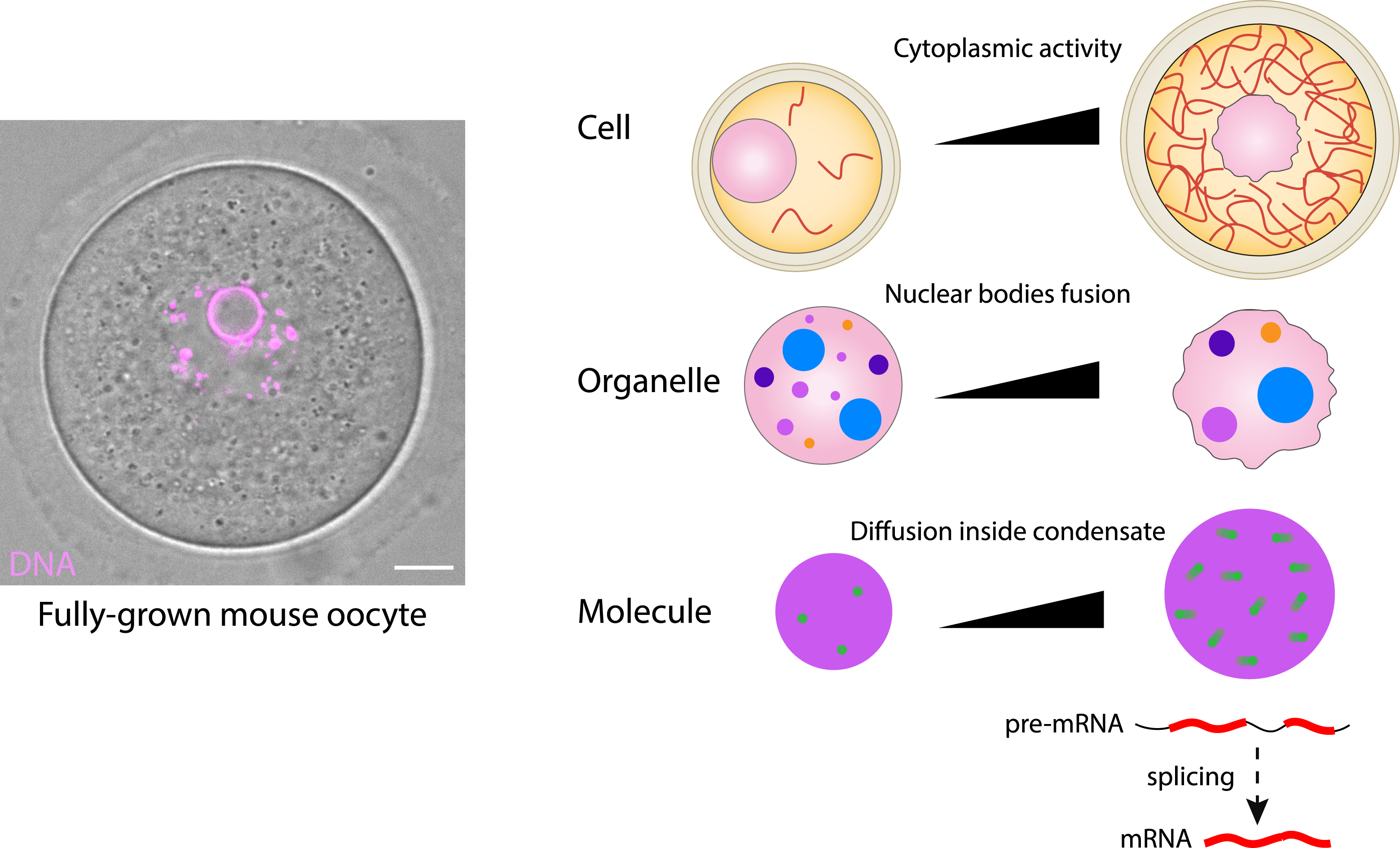
Our work in oocytes suggests that the cytoskeleton promotes transcriptional activity but also promotes mRNA processing and thus modulates the quantity and quality of maternal transcripts.
2. A thickening of the cortex prevents oocyte cortical contractions and preserves the architecture of organelles
A few decades ago, we also took an interest in studying the motion of the first meiotic spindle to the closest cortex, a prerequisite for the asymmetry in size of the first meiotic division (Figure 1, see arrow). Here again we demonstrated that biomechanics is instrumental is this process. The first meiotic spindle forms where the nucleus was, in the centre of the fully-grown mouse oocyte. Due to a high cytoplasmic viscosity, the spindle migrates towards the closest cortex along its long axis [23]. Indeed, the first meiotic spindle is never perfectly centered when it assembles, and often displays one of its two poles closer to one cortex. This initial slight imbalance in spindle position inside the oocyte is sufficient to break the symmetry in a cell that is otherwise non-polarized and with no obvious asymmetry [24]. Surprisingly, most oocytes are devoid of canonical centrosomes, made of a pair of centrioles surrounded by pericentriolar material [25]. Hence, they assemble their spindle a bit differently than cells in mitosis that use the two centrosomes to produce bipolar spindles (Figure 1). It takes about four hours in the mouse and eight hours in human to assemble a bipolar spindle [26, 27]. Importantly also, due to the lack of canonical centrosomes, meiotic spindles in oocytes are devoid of astral microtubules connecting the two spindle poles to the cortex. In somatic cells, these astral microtubules play key roles in controlling spindle orientation inside the cell, and in transmitting forces from the tissue towards the cell interior [28, 29, 30, 31].
In mouse oocytes, spindle positioning to the cortex cannot depend on astral microtubules, which are absent, but we and others have shown that it depends on F-actin [14, 15, 23, 32, 33]. Our lab has also shown that cortical properties, and in particular the thickening of the oocyte cortex during meiotic divisions is essential to amplify the initial imbalance of forces acting on meiotic spindle poles to promote first meiotic spindle off-centring [34, 35]. The cortical thickening depends on the nucleation of microfilaments via the Arp2/3 complex [34]. Recently, we used a conditional knockout of a key subunit of the Arp2/3 complex, Arpc4, to induce a depletion of this complex specifically in oocytes at the end of their growth [36]. This depletion was efficient in suppressing cortex thickening during oocyte divisions (Figure 5). Unexpectedly, Arp2/3 complex-depleted oocytes displayed important F-actin cortex reorganization (Figure 5 compare the two pictures), which subsequently triggered prominent contractions starting shortly after entry into meiosis. The energy of these cortex contractions, that lasted for a few hours, induced an increase in the motion of objects inside the cytoplasm and defects in the distribution and organization of different organelles, such as lipid droplets, mitochondria and cortical granules. Cortical granules have an important role in the prevention of polyspermy [37]. Not surprisingly, Arp2/3 complex-depleted oocytes displayed massive polyspermy, associated with poor embryo development after fertilization. As a consequence, female mice producing these mutant oocytes harboured a reduced fertility [38]. Interestingly, using an AI-pipeline [39] to automatically quantify oocyte morphological features on a collection of about 250 movies of developing human oocytes, we could observe that they presented similar cortex contractions, correlating nicely with increased cytoplasmic motion. However, in both mouse and human oocytes, the presence of cortex contractions during the first meiotic division did not correlate with aneuploidy, a major factor impacting the female gamete fitness [40, 41, 42]. The occurrence of contractions in developing human oocytes may correspond to a novel factor contributing to cytoplasmic infertility, different from the well-described aneuploidy. Previous studies have indicated a correlation between the developmental potential of oocytes and factors such as organelle size, quantity, and distribution, particularly concerning mitochondria, lipid droplets, and cortical granules [43]. In the future, we will investigate whether contracting human oocytes exhibit an altered organelle status, potentially linked to a reduced likelihood of successful development. While these oocytes are not used in routine in ART centres, certain patients may lack better alternatives. In such instances, the presence or absence of cortical contractions could aid in identifying oocytes with a higher probability of favorable outcomes [38] for these patients.
Arp2/3 depleted oocytes display contractions which impact organelles distribution. The absence of cortical microfilaments nucleated by the Arp2/3 complex in mutant oocytes without branched actin induces a reorganization of the oocyte cortex (compare bottom picture with top picture) and the appearance of cortical contractions. These cortical contractions promote the appearance of ectopic cytoplasmic flows, which impact on the movement and architecture of organelles (mitochondria, cortical granules). The progression of the oocyte cell cycle remains unaffected: oocytes exit normally from their arrest in prophase of the first division of meiosis (Prophase I), followed by normal progression of the first meiotic division (NEBD +2h and +6h) and an arrest in metaphase of the second division of meiosis (Metaphase II). However, fertilization of mutant oocytes is reduced, as is their early development. The fertility of mice producing these mutant oocytes is also reduced. The cortex is labelled (in fire) with a specific probe for F-actin.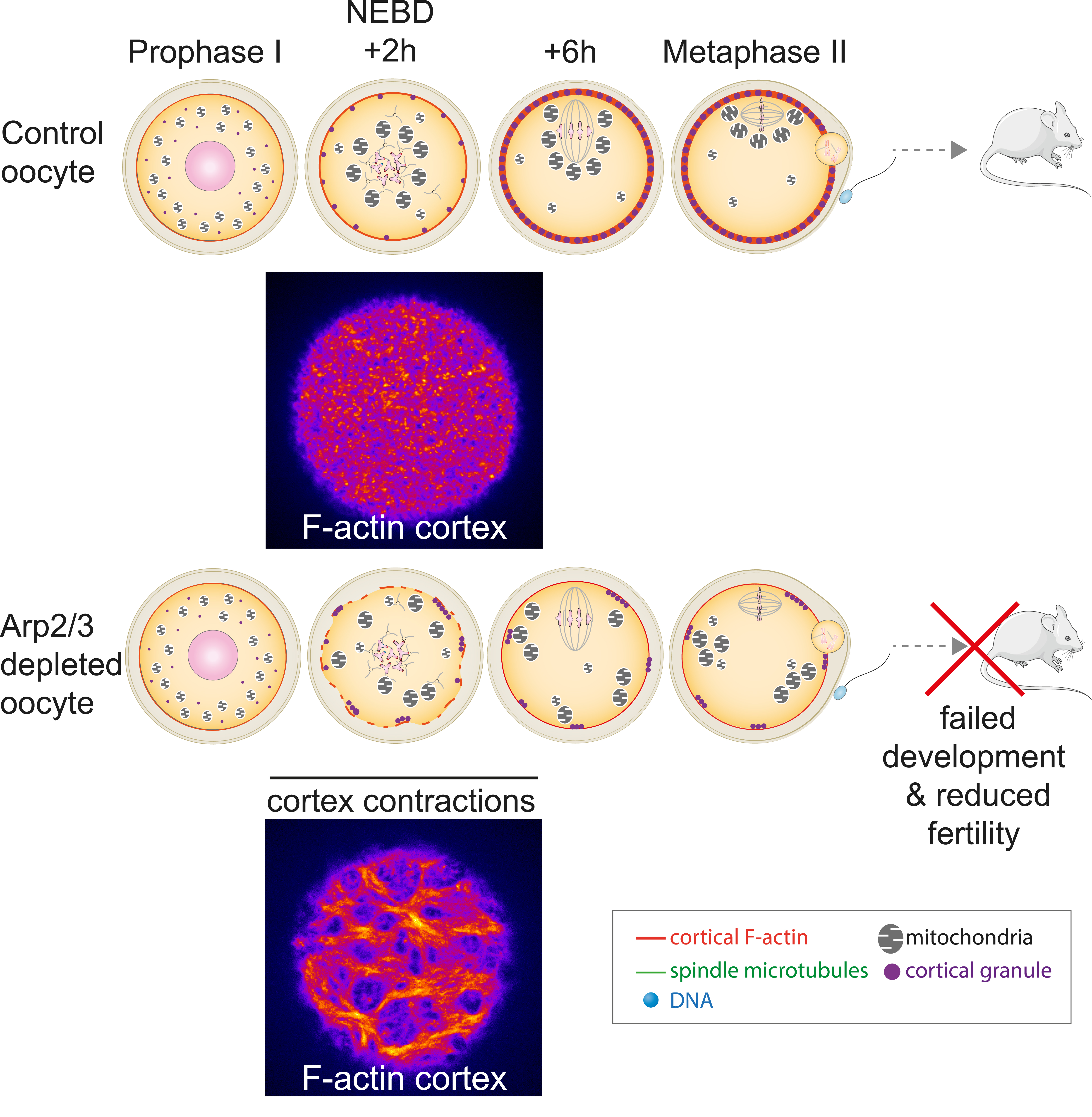
Altogether, our work has highlighted the impact of actin meshes in the control of maternal inheritance, in particular on chromosomes and organelle positioning, which are essential factors contributing to the developmental potential of the oocyte. Interestingly, both nucleus position and cortex properties constitute non-invasive predictors of early embryo development quality that could be used easily in Assisted Reproduction Technologies, where embryo selection currently consists of highly subjective morphological assessments.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.




 CC-BY 4.0
CC-BY 4.0