1 Introduction
The oogenesis of trematodes has been the subject of several studies by light [1,2] and electron-microscope [3–19]. The vitellogenesis of trematodes has already been studied for years [20–32], especially since Yosufzai [1] states that the egg-shell is formed by Mehlis gland reinforced by vitelline granules which serve as nutriment. The female reproductive system of Platyhelminthes shows great morphological variability with significant differences in the anatomical organization and cell structure by taxon [33]. Platyhelminthes were subdivised into two levels of organization, on the basis of the female reproductive system [34]. The Archoophora possess homocellular female gonads consisting of only germaria with oocytes, which produce both yolk and eggshell-forming precursors. Digenea are part of Neoophora, characterized by heterocellular female gonads composed of germaria (ovary) and vitellaria (vitelline gland). Each has a role in the egg formation [33]. Metadena depressa belongs to the Cryptogonimidae family (Ward, 1917). The Cryptogonimidae is a large family of Platyhelminthes found globally in the intestine or pyloric caeca of marine and freshwater teleosts, reptiles and, rarely, amphibians. The present study shows, for the first time, the ultrastructural composition of a Cryptogonimidae female reproductive system. The aim of the study is to describe the ultrastructural composition of germ cells, during oogenesis, and vitellocytes during vitellogenesis. The use of Thiéry method allows us to situate polysaccharides and glycogen granules [35].
2 Materials and methods
Adult specimens of M. depressa (Stossich, 1883) Linton, 1910 were collected live from the intestine of naturally infected common dentex Dentex dentex (Linné, 1758), caught off Valinco gulf, Corsica (Mediterranean Sea).
Worms were removed from their hosts, fixed in cold (4 °C) 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.2, rinsed in 0.1 M sodium cacodylate buffer at pH 7.2, post-fixed in cold (4 °C) 1% osmium tetroxide in the same buffer for 1 hour, dehydrated in ethanol and propylene oxide, embedded in Spurr and polymerized at 60 °C for 24 hours.
Ultra-thin sections (60–90 nm) of the worms, at the level of vitelline glands and the level of ovary, were cut on an ultramicrotome (Power tome PC, RMC Boeckeler). Sections were placed on 300 and 200-mesh copper and gold grids.
Sections on copper grids were stained with uranyl acetate and lead citrate (Reynolds, [36]). Sections on gold grids were stained with periodic acid, thiocarbohydrazide and silver proteinate (Thiéry, [35]). This technique was employed for the location of the glycogen.
Sections were examined on a Hitachi H-7650 transmission electron-microscope, operating at an accelerating voltage of 80 kV, in the “Service d’étude et de recherche en microscopie électronique” of the University of Corsica (Corte, France).
3 Results
3.1 Vitellogenesis
The paired vitelline glands of M. depressa consist of two lateral groups of vitelline follicles, distributed in ventral region, and may extend from testes to level of pharynx. Each follicle contains vitelline cells at various stages of maturation (Fig. 1). No muscle fibres were observed around vitelline follicles. Mature cells tend to be located toward the center of the follicle, but cells in various stages of development can be found anywhere in a follicle. Also, we can observe interstitial cells surrounding the developing vitelline cells (Fig. 2). Mitochondria, endoplasmic reticulum and glycogen granules occur through these cells (Fig. 2). No nucleus of these interstitial cells has been observed within vitelline follicles.
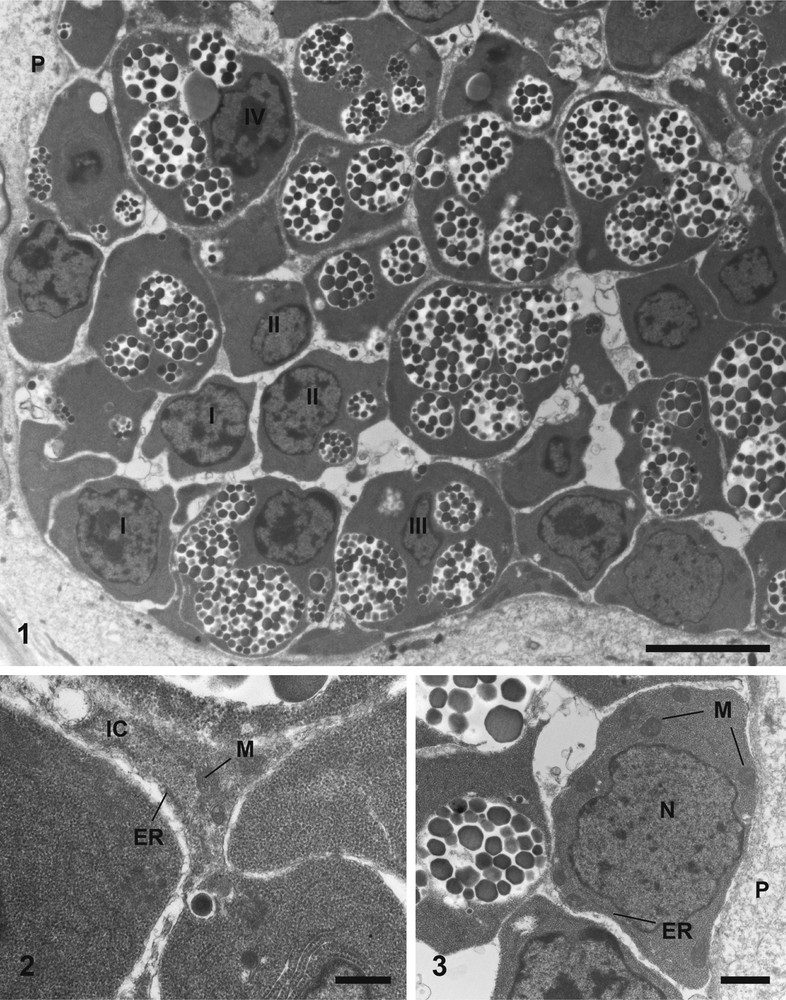
Electron micrographs of a vitelline follicle and the first stage of vitellogenesis, of Metadena depressa, stained according to Reynolds. (1) A vitelline follicle, which contains cells at various stage of development (I, II, III and IV). Bar = 4 μm. (2) The interstitial cells with mitochondria and endoplasmic reticulum. Bar = 0.5 μm. (3) An immature vitellocyte (stage I) at the periphery of the follicle, containing mitochondria and endoplasmic reticulum. Bar = 1 μm. ER: Endoplasmic Reticulum; IC: Interstitial Cells; M: Mitochondrion; N: Nucleus; P: Parenchyma.
Four stages of development have been observed from the immature cells to mature ones.
3.1.1 Stage I (Figs. 3, 4, 24A)
Immature vitellocytes of M. depressa have different shapes. They have a large, irregularly roundish nucleus, with diffuse chromatin scattered through the nucleoplasm (Fig. 3). In the earliest stages, they possess a high nucleo-cytoplasmic ratio (Fig. 3). In fact, they have little cytoplasm, filled predominantly with free ribosomes. The cytoplasm contains mitochondria with distinct cristae (Fig. 3), and elongated endoplasmic reticulum sacculi (Fig. 3). At this stage of development, these cells are devoid of glycogen (Fig. 4).
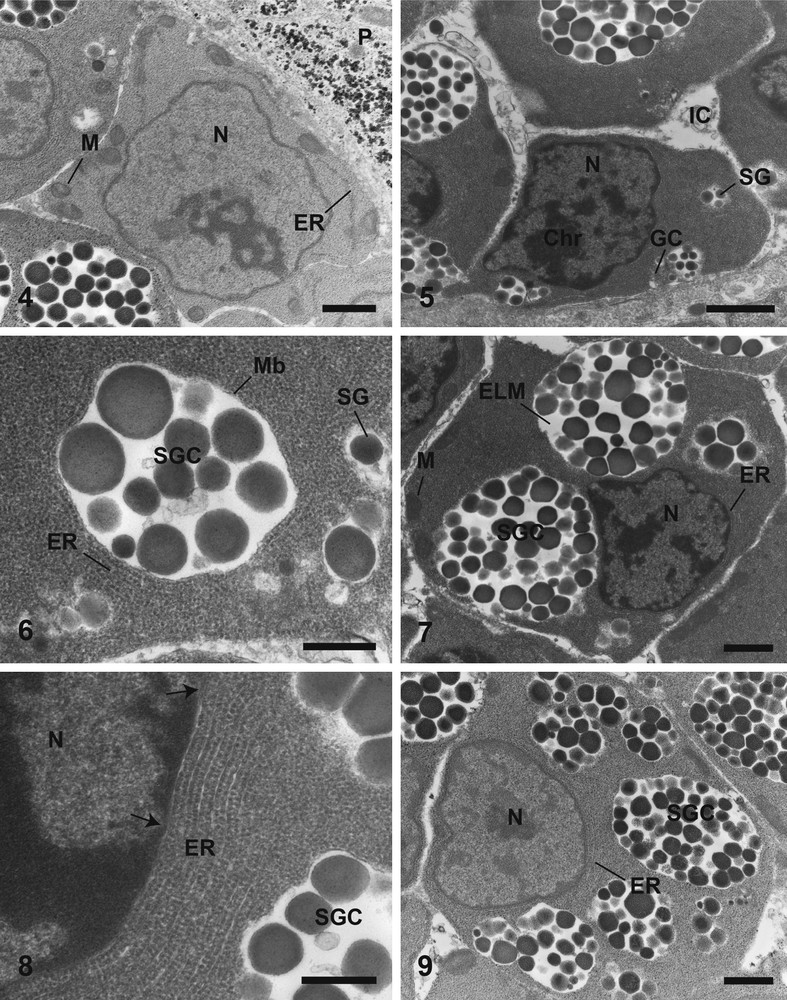
Electron micrographs of the first (4), the second (5, 6), and third (7–9) stages of maturation of vitelline cells stained according to Reynolds (5–8) and Thiéry (4, 9). (4) Cytoplasm of a cell at the first stage of maturation filled with ribosomes, with few endoplasmic reticulum and mitochondria, without glycogen granules. Bar = 1 μm. (5) Vitelline cell at the second stage of maturation. Bar = 1 μm. (6) Cytoplasm of a cell at the second stage of maturation showing the synthesis of a shell globule cluster from single shell globules. Bar = 0.5 μm. (7) General observation of a vitelline cell at the third stage of maturation. Bar = 1 μm. (8) Cytoplasm of a cell at the third stage of maturation with endoplasmic reticulum in parallel around the nucleus, and occurrence of nuclear pores (arrows). Bar = 0.5 μm. (9) Third stage of vitellogenesis without glycogen granules. Bar = 1 μm. Chr: Chromatin; ELM: Electron-Lucent Matrix; ER: Endoplasmic Reticulum; GC: Golgi complex; IC: Interstitial Cells; M: Mitochondrion; Mb: Membrane of shell globule cluster; N: Nucleus; P: Parenchyma; SG: Shell Globule; SGC: Shell Globules Cluster. Masquer
Electron micrographs of the first (4), the second (5, 6), and third (7–9) stages of maturation of vitelline cells stained according to Reynolds (5–8) and Thiéry (4, 9). (4) Cytoplasm of a cell at the first stage of maturation filled ... Lire la suite
3.1.2 Stage II (Figs. 5, 6, 24B)
The vitelline cells of the second stage have a nucleus including electron-dense patches of chromatin (Fig. 5). The chromatin is more condensed than in the earlier stage. The cytoplasm still contains mitochondria. The main difference compare to the earlier stage is the occurrence of shell globules (Figs. 5, 6). Golgi complexes occur near the shell globules (Fig. 5). Sometimes, shell globules are grouped in clusters and are surrounded by membrane (Fig. 6). These clusters continue their enlargement by fusion with single shell globules, and they are surrounded by endoplasmic reticulum (Fig. 6). As in the first stage, no glycogen has been observed in the early developing cells.
3.1.3 Stage III (Figs. 7–9, 24C)
During the maturation the chromatin is more condensed and larger than previously (Fig. 7). The nucleo-cytoplasmic ratio decreases along the vitelline cell development. In the maturing vitelline cells, each cluster contains an electron-lucent matrix, which increases along its development (Fig. 7). Moreover each shell globule cluster possesses an increasing amount of shell globules. There are few mitochondria (Fig. 7). At this stage, numerous endoplasmic reticulum sacculi are arranged in parallel around the nucleus or the edge of the cells (Fig. 8). Some nuclear pores are observed (Fig. 8). No glycogen granules are observed at this stage of maturation (Fig. 9).
3.1.4 Stage IV (Figs. 10–13, 24D)
Mature vitelline cells are generally localized in the center of the follicle. The nucleo-cytoplasmic ratio is the lowest of the vitelline cell development. There are large shell globule clusters arranged at the periphery of the cell (Fig. 10). There are approximately 45 globules per cluster and the average diameter of a cluster is 3 μm. Endoplasmic reticulum are less numerous than at the earlier stage but some residual sacculi remain around the nucleus and at the periphery of the cell (Fig. 11). Usually, the nucleus possesses a nucleolus (Fig. 12) and has a roundish shape but is not necessarily located in the center of the cell. In the most advanced stages, shell globules are surrounded by a large electron-lucent matrix in the clusters (Fig. 10). There are very few mitochondria, and most cells are devoid. The main feature of this mature stage is the apparition of lipid droplets (Figs. 10, 11). One to three lipid droplets were observed in mature vitelline cells. At this stage of vitellogenesis, glycogen appears in the cytoplasm. Glycogen granules are grouped in small clusters at the periphery of the cell, around lipid droplets and shell globule clusters (Fig. 13).
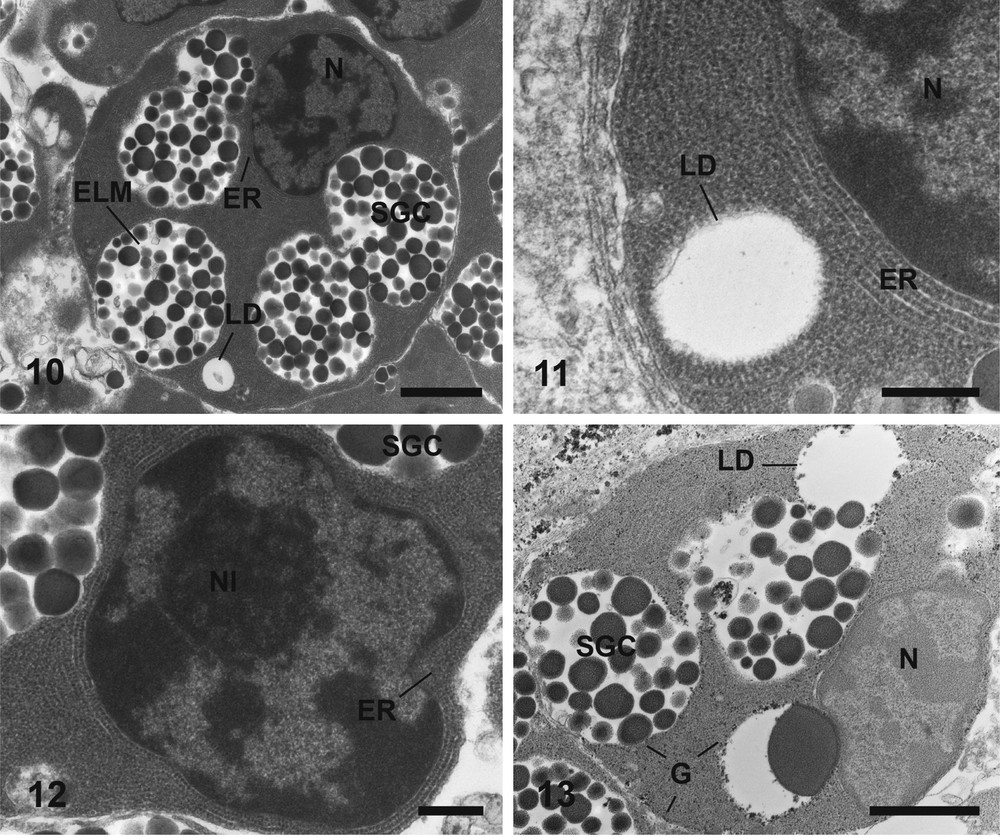
Electron micrographs of the fourth stage of maturation of vitelline cells stained according to Reynolds (10–12) and Thiéry (13). (10) General observation of a vitelline cell at the fourth stage of maturation, containing shell globule clusters, lipid droplet, with a very condensed chromatin in the nucleus. Bar = 2 μm. (11) A part of the cytoplasm with a lipid droplet and endoplasmic reticulum in parallel shape. Bar = 0.5 μm. (12) A nucleus of the fourth stage of maturation vitelline cells with a nucleolus, surrounded by endoplasmic reticulum. Bar = 0.5 μm. (13) Glycogen granules packed by heap around lipid droplets, the shell globule clusters, and at the periphery of the cell. Bar = 1.5 μm. ELM: Electron-Lucent Matrix; ER: Endoplasmic Reticulum sacculi; G: Glycogen granules; LD: Lipid Droplet; N: Nucleus; Nl: Nucleolus; SGC: Shell Globules Cluster. Masquer
Electron micrographs of the fourth stage of maturation of vitelline cells stained according to Reynolds (10–12) and Thiéry (13). (10) General observation of a vitelline cell at the fourth stage of maturation, containing shell globule clusters, lipid droplet, with a ... Lire la suite
3.2 Oogenesis
Ovary of M. depressa is an organ deeply lobed, located slightly anterior to testes. It contains germ cells at various stage of development, with younger cells localized in the periphery of the ovarian lobes. The oogenesis is divided into three main stages: oogonia, primary oocytes, mature oocytes.
3.2.1 Oogonia (Figs. 14, 25A)
The oogonia are small cells located at the periphery of the ovary, close to the basal lamina. These cells are irregular in shape and possess a small amount of cytoplasm. The nucleus is large and contains small patches of heterochromatin (Fig. 14). The cytoplasm is filled with free ribosomes, and contains few mitochondria and little smooth endoplasmic reticulum sacculi (Fig. 14).
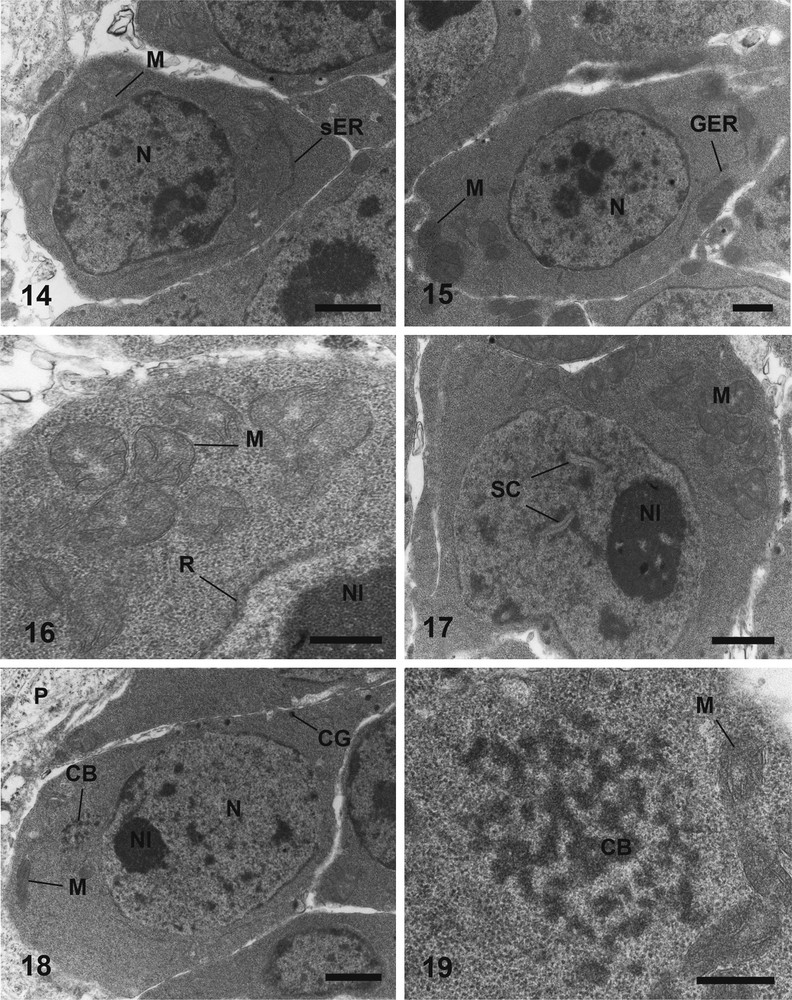
Electron micrographs of oogonia and oocytes maturation, of Metadena depressa, stained according to Reynolds. (14) Oogonium showing a little amount of cytoplasm, filled with free ribosomes, few mitochondria and smooth endoplasmic reticulum. Bar = 1 μm. (15) Primary oocytes with a large nucleus containing condensed heterochromatin. Bar = 1 μm. (16) Cluster of mitochondria in the cytoplasm of primary oocyte; note the ribosomal layer surrounding the nucleus. Bar = 0.5 μm. (17) Synaptonemal complexes indicating the zygotene-pachytene stage of the first meiotic division. Bar = 1 μm. (18) Maturation of oocyte, showing the beginning of cortical granules synthesize. Bar = 1 μm. (19) Chromatoid body surrounded by clusters of mitochondria. Bar = 0.5 μm. CB: Chromatoid Body; CG: Cortical Granules; GER: Granular Endoplasmic Reticulum; M: Mitochondrion; N: Nucleus, Nl: Nucleolus; P: Parenchyma; R: Ribosomal layer surrounding the nucleus; SC: Synaptonemal Complex; sER: smooth Endoplasmic Reticulum. Masquer
Electron micrographs of oogonia and oocytes maturation, of Metadena depressa, stained according to Reynolds. (14) Oogonium showing a little amount of cytoplasm, filled with free ribosomes, few mitochondria and smooth endoplasmic reticulum. Bar = 1 μm. (15) Primary oocytes with a large ... Lire la suite
3.2.2 Primary oocytes (Figs. 15–18, 25B)
The primary oocytes are also located in the distal germinative area of the gonad. The nucleo-cytoplasmic ratio is slightly lower than in oogonia (Fig. 15). Primary oocytes contain numerous mitochondria and a few granular endoplasmic reticulum, which are dispersed evenly in the cytoplasm (Figs. 15, 16). The nucleus is large and possesses condensed heterochromatin (Fig. 15) and a large nucleolus. The most mature primary oocytes show an enlargement of their nucleus, which possesses nuclear pores and a well-developed nucleolus (Fig. 17). Furthermore these germ cells show singulars characteristics with appearance of synaptonemal complexes in the nucleoplasm (Fig. 17) indicating the zygotene-pachytene stage of the first meiotic division. Some ribosomal material and granular endoplasmic reticulum sacculi surround the nuclear membrane in the ooplasm (Fig. 16). At this stage, germ cells tend to detach from the basal lamina.
3.2.3 Mature oocytes (Figs. 19–24, 25C)
Mature oocytes are often located in the central area of the ovarian lobe. The nucleo-cytoplasmic ratio decreases along the maturation due to the remarkable increase in the volume of the ooplasm (Figs. 18, 22). The nucleus possesses a nucleolus, smaller than in the earlier stage, which tends to disappear at the end of maturation (Figs. 18, 22). Small clumps of heterochromatin are present near the nuclear membrane (Figs. 18, 22). Conspicuous aggregates of non membrane-bound granular electron-dense material are observed in the cytoplasm (Figs. 18, 19, 22). This chromatoid body is often surrounded by mitochondria (Figs. 18, 22). Small vesicles, with an electron-dense content and an electron-lucent matrix enclosed within a smooth membrane, occur at this stage of oogenesis (Fig. 18). These membrane-bound granules (approximately 0.25 μm of diameter) are synthesized within the ooplasm (Fig. 20) and then migrate in monolayer to the periphery (cortex) of the cell (Figs. 20, 22). The number of cortical granules increases along the maturation (Figs. 18, 22). The cytoplasm contains many slender mitochondria (Fig. 21). These organelles tend to cluster into one or two groups in the cytoplasm (Fig. 22). The amount of mitochondria is high in fully mature oocytes (Fig. 22). Some granular endoplasmic reticulum sacculi are still observed (Fig. 22). Neither glycoprotein nor polysaccharides have been observed during all the stages of oogenesis (Fig. 23).
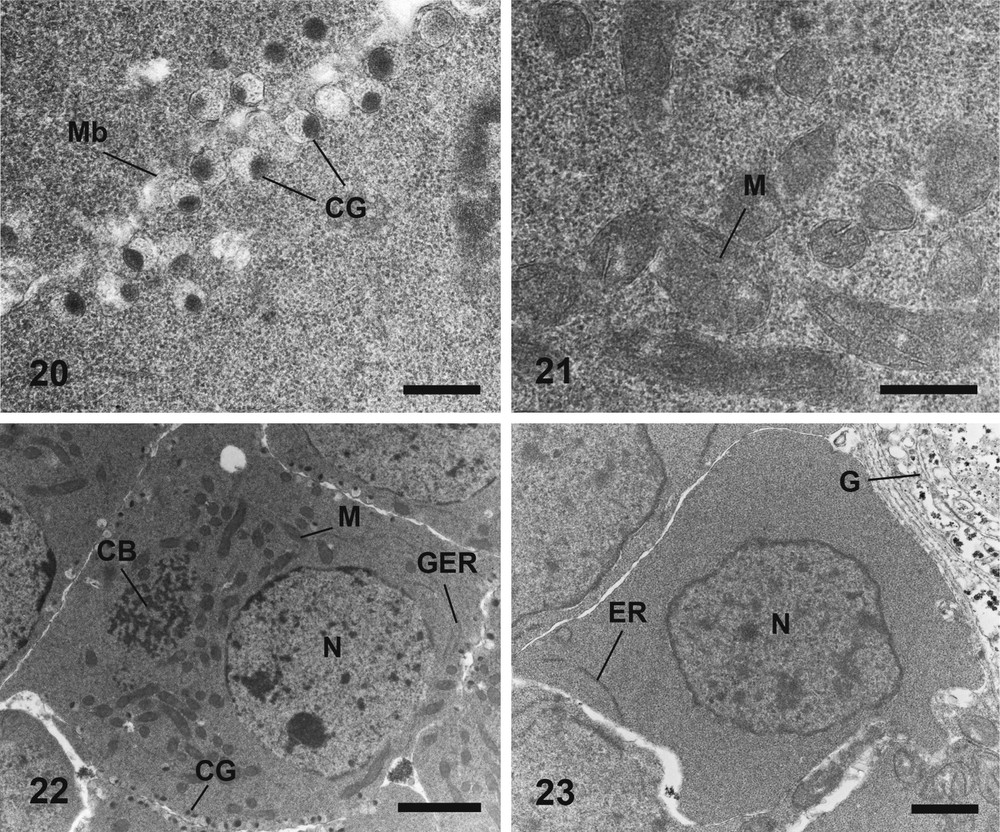
Electron micrographs of developing and mature stage of oogenesis, of Metadena depressa, stained according to Reynolds (20–22) and Thiéry (23). (20) Cortical granules at the periphery of two mature cells. Bar = 0.5 μm. (21) A cluster of mitochondria at a pole of a mature oocyte. Bar = 0.5 μm. (22) General observation of a fully mature germ cell. Bar = 2 μm. (23) General view of an oocyte at the first stage of maturation, without glycogen granules. Bar = 1 μm. CB: Chromatoid Body; CG: Cortical Granules; G: Glycogen granule in the parenchyma; GER: Granular Endoplasmic Reticulum; M: Mitochondrion; Mb: oocyte Membrane; N: Nucleus. Masquer
Electron micrographs of developing and mature stage of oogenesis, of Metadena depressa, stained according to Reynolds (20–22) and Thiéry (23). (20) Cortical granules at the periphery of two mature cells. Bar = 0.5 μm. (21) A cluster of mitochondria at a pole ... Lire la suite
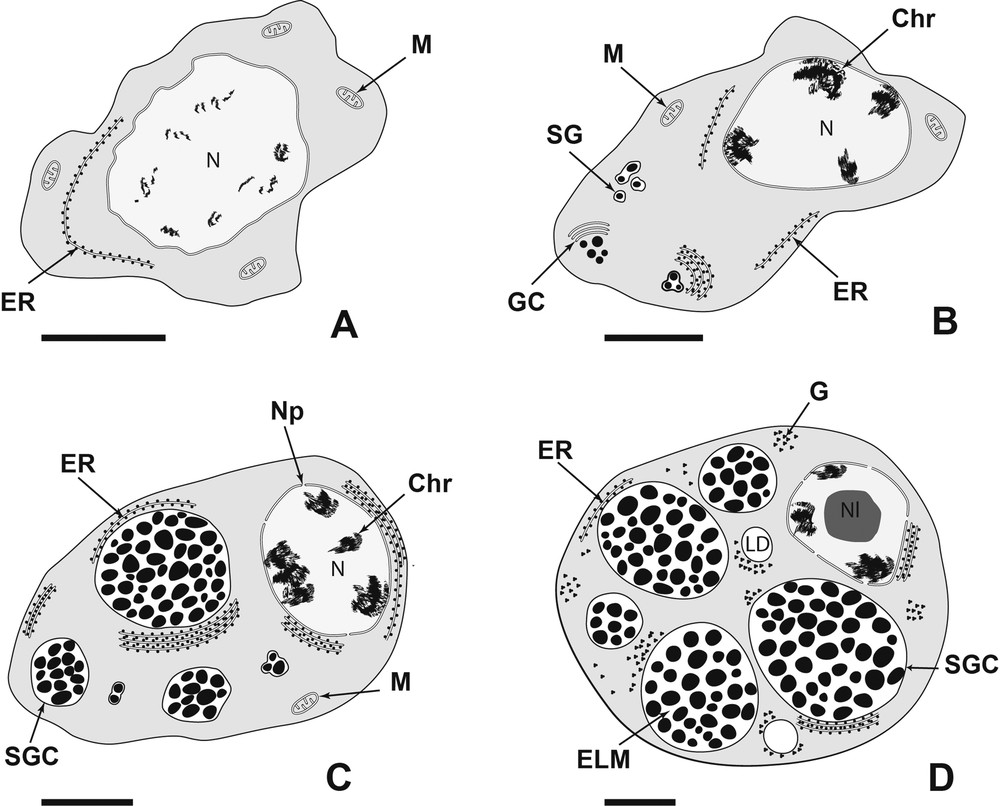
Diagram showing the four stages of vitellogenesis of Metadena depressa. (A): stage I; (B): stage II; (C): stage III; (D): stage IV. Bar = 2 μm. Chr: Chromatin; ELM: Electron-Lucent Matrix; ER: Endoplasmic Reticulum sacculi; G: Glycogen granules; GC: Golgi Complex; LD: Lipid Droplet; M: Mitochondrion; N: Nucleus; Nl: Nucleolus; Np: Nuclear pore; SG: Shell Globule; SGC: Shell Globules Cluster.
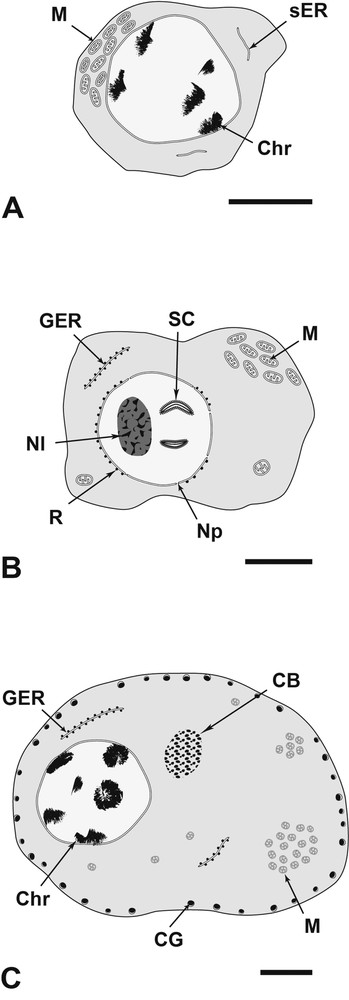
Diagram showing the three stages of oogenesis of Metadena depressa. (A): Oogonium; (B): primary oocyte; (C): mature oocyte. Bar = 2 μm. CB: Cytoplasmic Body; CG: Cortical Granule; Chr: heteroChromatin; GER: Granular Endoplasmic Reticulum; M: Mitochondrion; Nl: Nucleolus; Np: Nuclear pore; R: Ribosomal perinuclear material; SC: Synaptonemal Complex; sER: smooth Endoplasmic Reticulum.
4 Discussion
4.1 Vitelline cells
The maturation of vitelline cells of M. depressa is divided into four stages: stage I, cells are undifferentiated; stage II, beginnings of the synthetic activity; stage III, active shell globule clusters synthesis; stage IV, mature cells. Although the vitellogenesis is basically the same in all Digenea [16,20–29,33,37–39], some ultrastructural differences occur during the maturation, particularly with other Platyhelminthes [30,40–50]. A Cryptogonimidae vitellogenesis has already been studied, which allows us to compare the process [31]. The interstitial cells are believed to be involved in the selection and transport of materials from parenchyma to the developing vitellocytes [21,33]. Nevertheless, this structure is absent in some digenean vitelline follicles [16,23].
The nucleo-cytoplasmic ratio decreases along the vitellogenesis due to the synthesis of large cytoplasmic inclusions. In Dicrocoelium dendriticum, nucleoli have been observed from the first stage of maturation [27], whereas in M. depressa only fully mature vitellocyte possess a large nucleolus. No ‘degenerating nucleus’ [16], containing varying amounts of coarse granular material, have been observed. Every digenean nucleus does not possess a nucleolus at the ultimate stage of maturation in vitelline follicles, like Pharyngostomoides procyonis and A. tubarium [23,31].
The attendance of endoplasmic reticulum sacculi, free cytoplasmic ribosomes, and Golgi complexes in the second stage traduces a high synthetic activity [16,25]. This synthesis gives rise to shell globules, which coalesce to form clusters. A primary role of Neodermata vitelline cells is to produce this eggshell substance [51]. These cells will pass through the vitelloduct and be enclosed in a capsule with one or more fertilized egg [27]. The beginning of the eggshell globules synthesis is more precocious in M. depressa than in A. tubarium [31]. The shape and amounts of shell globules in clusters differ by species. In M. depressa, the electron-lucent matrix is greater than in A. tubarium and the amount of shell globules by cluster seems to be higher too.
The second role of digenean vitelline cells is to accumulate nutritive reserves for the future developing embryo [27,46]. The occurrence of lipid droplets in the fully mature vitellocytes and the glycogen content represent this nutrition reserves. A major difference occurs into Cryptogonimidae family, because several highly osmophilic [52] and electron-lucent lipid droplets are present in M. depressa most mature cells, as for Schistosoma-mansoni [21], whereas only one appears in A. tubarium. Nevertheless, both possess α- (particles) and β- (rosettes) glycogen [52–54] in the mature vitellocytes cytoplasm, unlike Halipegus eccentricus [16].
4.2 Oogenesis
On the basis of the female gonad structure Platyhelminthes are subdivised into two levels of organization. In neoophoran platyhelminths, the production of yolk and shell-forming precursors occurs in vitellaria, unlike in archoophorans where it takes place in germaria [34]. The initial phase oocytes maturation takes place during the prophase of the first meiotic division in the ovary. This maturation has been subdivided into three stages, as for other Digenea [7,13,17,34], Monogenea [55], and Cestoda [56]. Despite these oogenesis is basically the same in all Platyhelminthes, some ultrastructural differences occur during the maturation of oocytes.
The interstitial cells were thinner than in other digenetic trematodes [12,15]. The oogonia were observed along the wall of the ovary. According to Nez and Short [2] in Schistosomatium douthitti, the secondary oogonia are pushed toward the center of the ovary. In the present study, only primary oocytes were pushed to the periphery of the germarium. As for Cryptocotyle lingua, in M. depressa a definite line of demarcation of the oogonia from the oocytes does not exist [18]. They have been distinguished by their size and organelles content. Few inclusions are founded in the cytoplasm, excepted mitochondria, which are grouped in a pole of the cell and patch of endoplasmic reticulum sacculi. By unknown number of mitotic divisions, oogonia differentiate into primary oocytes.
Then, primary oocytes enter the zygotene-pachytene stage of the first meiotic division recognizable by the presence of synaptonemal complexes in the nucleoplasm. The metaphase takes place in the proximal part of the uterus where the fertilization occurs [19]. The presence of nuclear pores and granular material in the perinuclear region, in particular near the nucleolus, suggests a mass transfer to the cytoplasm [8,9]. This granular material tends to coalesce and form a non membrane-bound chromatoid body. This phenomenon has been commonly described in primary oocytes [6,8,9,57], and variously named (e.g. ‘nuclear extrusion’, ‘nucleolus-like cytoplasmic body’). This granular mass is commonly surrounded by clustered mitochondria, excepted for Dicrocoelium dendriticum where cisternae of endoplasmic reticulum and Golgi complex surrounded this structure [13]. Burton [6] considered that the process is probably associated with the migration of RNA to the cytoplasm, and Koulish [9] underline that this granular structure contains RNA [11]. No Golgi complexes have been observed in the present study, like in Fasciola hepatica [7], even though the cytoplasmic inclusions are certainly synthesized by endoplasmic reticulum sacculi and Golgi complexes.
In the fully mature oocytes, we have observed the main synthesized inclusion of the oogenesis. Small vesicles, initially randomly distributed in the cytoplasm, migrate to the cortical cytoplasm where they form a monolayer just beneath the oolemma. These vesicles have been described in numerous digenean trematodes [3–15], and in other Platyhelminthes [45,50,51,55,58–76]. The chemical composition of these vesicles is not already known. For Cifrian et al. [13] and Gupta et al. [10] cortical granules consist of proteins and glycoproteins and do not contain polyphenols; however it is the opposite in some other Platyhelminthes [64]. In the present study, the TCH incubation [35] made us conclude to the lack of polysaccharides and glycogen in germ cells of M. depressa. Nevertheless these granules should function similar to those in oocytes of other animals, which act in the blocking of polyspermy during fertilization [15]. Some cestodes studied to date are devoid of these cortical granules [77].
Some concentric loops of endoplasmic reticulum sacculi surrounding small electron-dense aggregates have been described in D. dendriticum oocytes [13], whereas they have been observed in M. depressa vitellocytes.
The amount of mitochondria is important in mature oocytes, and they are clustered in one or two groups at a pole of the mature germ cells. The distribution of mitochondria is correlated with the phases of growth and development of the oocytes and is no doubt related to their functional activity [8]. Fully mature oocytes of M. depressa are devoid of lipid droplets in the ooplasm, unlike Halipegus eccentricus [14]. This characteristic could result from the important lipid content of vitellocytes of M. depressa, which provides enough lipids for the developing embryo. It is probable that the process of oogenesis of M. depressa is not over when the oocyte has left the ovary.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


