1. Mesoamerican agriculture
The development of agriculture is arguably the most significant element of the so-called Neolithic package [1]. This package includes the Neolithic Revolution [2] with the domestication of plants and animals, technological advancements, and the shift to sedentary lifestyles, along with the accompanying economic, social, and ideological changes that led to the emergence of complex societies [3]. The transition to agrarian societies independently arose in different regions across the globe, recognized as centers of domestication [4]. This shift was likely in part driven by major climate changes at the end of the Pleistocene, which included a reduction in climate variability, a rise in atmospheric CO2 levels, and an increase in rainfall, all of which dramatically transformed the Earth’s environment [5]. One well-documented example is the Mesoamerican domestication center, which refers to the pre-Columbian cultural region encompassing central Mexico to the western regions of Honduras, Nicaragua, and Costa Rica [6]. Several archaeological discoveries in Mesoamerica attest to human occupation in the region at the late-Pleistocene and early-Holocene [7], with the earliest evidence found at Chiquihuite cave in the state of Zacatecas in Mexico dating from the last glacial maximum (26,500–19,000 yr BP) [8]. Cave excavations in Mexico indicated that between the sixth and the five millennia BP, survival was ensured by fishing, megafauna hunting, and plant gathering activities [9]. A progressive increase of food production along with an increased sedentarization between 10,000 and 3000 yr BP is documented in Mesoamerica [10, 11].
Mesoamerica was the cradle of major staple crops, including among other species maize (Zea mays ssp. mays L.) [12], multiple bean species (Phaseolus spp.) [13], multiple squash species (Cucurbita spp.) [14, 15], avocado (Persea americana Mill.) [16], and chili peppers (Capsicum annuum L.) [17]. Beyond its role in social and economic development in Mesoamerica, agriculture was and still is deeply intertwined with cultural diversity in the region.
Maize, in particular, was revered as a sacred plant by Mesoamerican societies, as evidenced by the etymology of maize’s wild ancestor in Nahuatl—the language spoken by the Aztecs—“teocintle” meaning “sacred maize ear” [18, 19]. Analyses of jade caches in Maya temples of Guatemala indicated the central role of the “Maize God” in sacred rituals [20]. In various ethnic groups in Mesoamerica, maize is anthropomorphized and believed to possess a sacred soul (Figure 1). The significance of maize beliefs among different ethnic groups in Mesoamerica (Teenek, Nahua, Xi’iuy and Totonac) was also highlighted [18]. Moreover, as a central element of the local populations’ diets, maize has been and continues to be the subject of numerous pilgrimages and prayers to ensure successful harvests [21].
Milpa mythology. Mural painted by Fernando Castro Pacheco in 1971 at the Palacio de Gobierno in Mérida (Yucatán, Mexico), embodies the Mayan mythology around maize. At its center is a Mayan man emerging from the fertile ear of maize, held in the hand of the southern divinity (bacab) as depicted in the “Popol Vuh”, the sacred book of the Maya. Overseeing this scene is the northern bacab, symbolized by a white hand at the top. Both the western (left) and eastern (right) bacabs contemplate their creation, represented by the rich foliage of the maize plant, symbolizing the essence of life (Photo by M. Tenaillon).
Archaeological remains show that agricultural systems developed in Mesoamerica were diverse and sophisticated. Mesoamerican farmers developed techniques such as irrigation, terracing, crop rotation and mixed cropping [22, 23]. Perhaps the most emblematic intercropping system is the tripartite milpa system, often composed of squash (Cucurbita spp.), common bean (Phaseolus vulgaris L.)—hereafter bean—and, maize. The word “milpa” originates from the Nahuatl language, where “milli” means planted plot and “pan” means upon [24].
The milpa was likely developed in the Guerrero-Jalisco area, in the Western part of Central Mexico, where agri-food technology became highly advanced between 4500 and 3500 yr BP. This is evidenced by the use of clay pots for steaming and cooking, technologies that were not present in other parts of Mesoamerica at the time [25]. Milpa subsequently became the cornerstone of pre-Columbian agriculture, spanning a vast region from northeastern North America [26] to northern South America [27]. The complementary nutritional intake provided by the three crops likely contributed to its widespread success [25].
As it spread and was adopted through Mesoamerica and beyond, the milpa system diversified in various ways. First, in terms of terminology, milpa is referred to as “Diohe’ko”—things that sustain us—in Seneca language [26, 28], as “quèela”, “yela” or “yel” in Zapotec dialects [29] or “ñaa” [30] while the Mayans designate it as “Kool” [31]. Second, milpa was also the basis of a rich diversity of mythology and beliefs. For the Iroquoian people, the three plants were considered as a sacred gift as they miraculously sprouted from the body of Sky Woman’s daughter, thus granting the gift of agriculture to the Iroquois nations [32]. Third, the milpa system diversified through the inclusion of various associated crops, such as other species of the Phaseolus genus (Phaseolus lunatus L., Phaseolus acutifolius A. Gray and, more rarely, Phaseolus coccineus L. and Phaseolus dumosus Macfadyen—see [27]), agaves (Agave ssp.), chilis (Capsicum spp.), husk tomatoes (Physalis spp.), chan (Hyptis suaveolens [L.] Poit), hog plums, jocote (Spondias purpurea L.) among other species [25, 33].
In this review, we first detail the domestication processes of maize, beans, and squash, and the subsequent development of the milpa system in the Guerrero-Jalisco region. We then examine how milpa, a traditional agri-food system, was adapted by small-scale European farms and later integrated into modern agricultural practices. Finally, we highlight the beneficial interactions between these three crops, emphasizing their potential as key elements in promoting agroecology.
2. The emergence of milpa in the Guerrero-Jalisco area
Archaeological findings and genetic data indicate that the simultaneous growing of the three crops appeared not very long after their respective domestication [34, 35, 36]. In fact, it is possible that the wild relatives of maize, bean and squash were consumed together even before domestication in the pre-ceramic period, as evidenced by contemporary consumption of wild forms using tools and techniques from the Archaic time period in Mesoamerica [25]. The co-occurrence of the wild and early-domesticated forms of the three taxa in southwestern Central Mexico would have probably facilitated their association (Figure 2). Interestingly, the domestication of the three crops tells very different stories. These differences are rooted in the distributions of their wild relatives and are further shaped by biological differences. Maize, for example, is a monoecious, outcrossing and wind-pollinated plant. In contrast, common beans are predominantly self-pollinating, while squash species, which are mostly monoecious, rely on insect pollination for outbreeding. Moreover, human-directed selection has resulted in distinct domestication syndromes for each species.
Domestication areas, routes, and introductions into Europe of the three crops composing milpa. In the figure (left panel), colors indicate each species. In gold, maize was domesticated in Mesoamerica around 9000 years before present (yr BP). Maize spread northward and southward across the American continent before being introduced to southern Spain following Christopher Columbus’ expeditions in 1492 and to northern Europe before 1539. In orange, squash (C. argyrosperma) was domesticated in Mesoamerica around 10,000 yr BP, but little is known about the routes it took before being introduced to Europe. In green, beans underwent two main independent domestication events around 8000 yr BP in Mesoamerica and the Andes, resulting in two distinct gene pools: M1 and A2. A secondary domestication from M1 led to the M2 group, while two secondary domestication events from A2 led to the A1 and A3 groups. All groups, except A2 due to its photoperiod sensitivity, were introduced into Europe after Pizarro’s explorations in 1529 for the Andean groups. Today, intercropping of maize-bean-squash is still practiced in some traditional European farming systems (right panel, photo from southwestern France by Maud Tenaillon).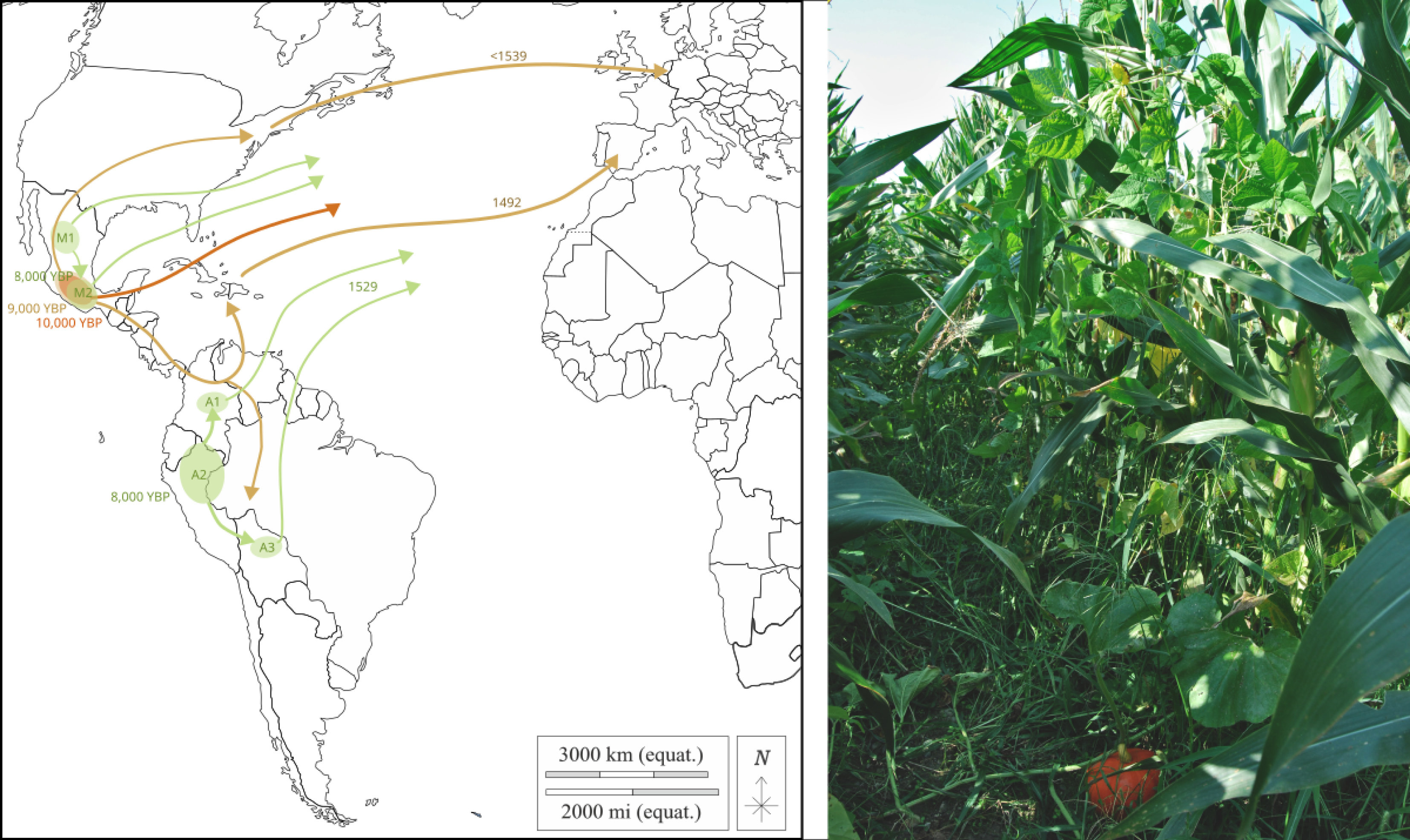
Maize (2n = 2X = 20) was domesticated in the Rio Balsas drainage basin located in northern Guerrero (Figure 2) from the annual subspecies of teosintes, Zea mays ssp. parviglumis around 9000 yr BP [37, 36, 12]. Recent results have also found that ssp. mexicana has also greatly contributed to maize make-up through admixture—the genetic mixing of these two subspecies through interbreeding [38]. Many traits differentiate maize from teosintes (reviewed in Tenaillon and Manicacci [39]). A major one is the softer glume, which leads to kernel exposition and accessibility, making maize a valuable crop [40]; starch properties were also modified in maize [41]. Regarding the architecture, the prevention of axillary meristem development led to the suppression of the elongation of lateral ear branches [42, 43, 44], which translated into strong apical dominance and short ear-tipped branches in maize [34, 45]. Additionally, domestication has resulted in increased yield with dramatic increase in female ear length and width [46], as well as a reduction in seed shattering [47].
The genus Phaseolus (2n = 2X = 22) is composed of 70 species. Among them, five are domesticated including P. vulgaris (common bean), which is by far the one with the broadest geographic distribution. This species was domesticated twice independently [48]. The wild species P. vulgaris originated in Mexico and spread into South America, leading to two distinct and geographically isolated wild gene pools in Mesoamerica and the Andes [49, 50]. Domestication occurred independently in these gene pools around 8000 yr BP [51], with ongoing debate about the precise locations (Figure 2). Mesoamerican domestication is supported by both molecular and archaeological evidence that pinpoint the Rio Lerma–Rio Grande de Santiago basin in Jalisco, in West Central Mexico [52]. However, nucleotide data suggest an earlier domestication in the Oaxaca Valley in southern central Mexico [53], an area where maize also spread early through human migration [54]. A recent study proposes that initial domestication has occurred in high-altitude regions of Jalisco and Durango (Figure 2, M1), with a secondary domestication of low-altitude races (Figure 2, M2) in Mesoamerica [55]. In the Andean gene pool, potential domestication sites include central-southern Peru based on chloroplast DNA [48], while nuclear nucleotide data and archaeological findings from Huachichocana in Argentina suggest southern Bolivia and northern Argentina [56]. Recent findings indicate a primary domestication in Peru (Figure 2, A2), followed by two secondary domestications (Figure 2, A1 and A3), defining three distinct Andean gene pools [55]. Multiple domestications may have been facilitated by the selfing mating system of this species. Domestication resulted in morphological changes including loss of pod dehiscence, traits limiting seed dispersal and seed dormancy, fewer nodes on the main stem, larger pods and seeds [57].
The genus Cucurbita (2n = 2X = 40) includes 14 species, with six independent domestications from various wild ancestors present across the Americas [58, 14, 59]. Kate et al. [60] established the first squash phylogeny based on and suggested different domestication centers: C pepo L. ssp. pepo (pumpkin) and C. argyrosperma Huber ssp. argyrosperma (cushaw pumpkin) in Mexico, C. moschata Duchesne (butternut squash or calabaza) in Colombia, C. pepo L. ssp. ovifera (L.) Harz (summer squash) in eastern North America, and C. ficifolia Bouché (figleaf gourd or chilacayote) and C. maxima Duchesne ssp. maxima (Hubbard squash) in South America. More recent results, however, suggest domestications of C. moschata and C. ficifolia in Mexico [14, 61, 62]. The domestication center of C. argyrosperma was pinpointed in the lowlands of Jalisco (Figure 2) using genomic data from 192 wild and domesticated individuals [15]. In milpa agriculture, commonly used Cucurbita species include C. pepo, C. moschata, C. argyrosperma and C. ficifolia [14]. The earliest archaeological evidence for C. maxima and C. argyrosperma dates back to 4000–5000 yr BP, while evidence for C. pepo dates back to 8000–10,000 yr BP [63]. The domestication of pumpkins and squashes in Mesoamerica likely began with human selection favoring less-bitter seeds [64]. Today, while all major Cucurbita crops are cultivated for their fleshy fruit, C. argyrosperma is still mainly valued for its seeds [15, 14]. The evolution of edible flesh involved the reduction of bitter compounds known as cucurbitacins [65]. Despite distinct primary usages, many domestication traits are common among the domesticated Cucurbita species. They include loss of bitter compounds, reduction of physical defenses such as urticating trichomes, elimination of seed dormancy, enlargement of fruits and seeds, and diversification of fruit morphology [15, 66, 67].
3. The milpa, from Mesoamerica to Europe
Maize spread northward into southwestern United States around 4000 yr BP [68]; and southward from Mexico (Figure 2), reaching southwestern Amazonia by 6500 yr BP [69]. Southwestern Amazonian basin may have acted as a secondary center of improvement before the subsequent diffusion of this “improved” maize to lowland South America east of the Amazonian basin and to the Andes in the west [70]. In Europe (Figure 2), Caribbean maize adapted to warm climates was introduced to southern Spain after Columbus’s 1492 expedition [71]. Flint maize from North America reached northern Europe, as noted by Bock in 1539 [72] and later confirmed by genetic studies [73]. Genetic analyses revealed that northern European landraces are genetically linked to their North American counterparts, while southern European landraces are closer to Caribbean lowland tropical maize [74, 75].
Similarly, P. vulgaris arrived in Europe following early European exploration of the Americas (Figure 2). All groups except A2, which is sensitive to photoperiod, were introduced to Europe [55]. The prevalence of Andean accessions and smaller Andean introgression segments in European varieties suggests that the Andean gene pool was introduced before the Mesoamerican gene pool to Europe [55], following Francisco Pizarro’s 1529 expedition to northern Peru [76].
As for Cucurbita species, there is currently limited knowledge about their spread outside their centers of origin (Figure 2).
Admixture played a crucial role in shaping specific genetic groups in Europe. In maize, similar to the emergence of Corn Belt dents in the mid-latitudes of the United States, European Flints likely originated from admixture between northern and southern European genetic materials [75, 73], adapting maize to European climates. In common beans, the distribution of genetic groups in Europe reflects their adaptive traits, particularly in terms of photoperiod sensitivity and climate adaptation. Hybridization between Andean and Mesoamerican P. vulgaris gene pools after introduction to Europe resulted in new allele combinations, evident in the higher level of admixture among European bean varieties [49]. However, the dissemination of common beans in Europe was influenced not only by local adaptation, but also by political and cultural factors [55].
The three crops (maize, bean, Cucurbita) spread in Europe following their introduction. The question of whether varieties of the three taxa used in multi-cropping systems were co-introduced to Europe at the time of the discovery of the Americas, as opposed to being re-associated later in Europe, remains open.
European farming practices resembled traditional Mesoamerican milpa systems, characterized by low-input field management and the use of traditional landraces. However, maize was (and still is) used differently: in Mesoamerica, tropical maize landraces underwent nixtamalization—a process of soaking and cooking maize kernels in an alkaline solution—before being ground into so-called masa dough for tortillas [77]. In Italy, and likely across Europe, maize, mostly of flint types, was typically ground into coarse meal for polenta. Maize was first mentioned on the Italian peninsula in 1495 and initially cultivated in the Veneto region, helping northern Italian farmers avoid food shortages and famine [78]. However, without the knowledge of the nixtamalization process, which releases niacin (vitamin B3) and essential amino acids, maize consumption leads to nutritional deficiencies. It is possible that the early adoption of the milpa system helped mitigate these deficiencies by providing nutritional complementarity. From the late 18th century onward, however, northern Italy saw a gradual shift toward a maize-based diet, which led to outbreaks of pellagra [78]. In contrast, in the Hautes-Pyrénées department in France, the coculture of maize and bean was common covering 18,500 hectares in the 19th century [79]. In this region, the combined cultivation of all three taxa was actually frequently found in gardens and small farms for subsistence farming [80]. Traditional multi-cropping farming practices were abandoned in Europe after the Green Revolution, largely due to the introduction of highly productive maize hybrids [81, 82].
Today, milpa in Europe is restricted to a few areas such as the Pyrenees (Figure 2), Transylvania and southern Italy [83], where maize landraces are selected for their flint type for polenta and bean is also used for self-consumption. Recently, there has been a renewed interest in integrating multi-crop farming into modern agricultural practices. This revival is evident in the maize-bean intercropping system adopted in the Tarbes region of France in the 1980’s [79], in the Potenza region of Italy (personal communication from Elisa Bellucci), and areas of Germany [84]. These systems involve combining bean cultivars with maize hybrids, often using chemical inputs. Maize in these modern setups is often utilized for animal feed.
4. Beneficial mechanisms of traditional milpa
Several studies have observed an overall performance advantage of the milpa system compared to crops grown alone, due to positive interactions. This is assessed using the Land Equivalent Ratio (LER) index, the most commonly used metric for evaluating land use efficiency in intercropping systems relative to sole-cropping [85]. Findings consistently show that milpa systems have LER values greater than 1 compared to monocultures, indicating superior land use efficiency of the mixture, a phenomenon also known as overyielding [86, 87, 88, 89].
The overyielding observed in milpa systems can be attributed to two essential non-exclusive beneficial mechanisms underlying positive interspecific interactions: niche partitioning and facilitation [90]. On the one hand, niche partitioning maximizes the exploitation of light and soil resources through spatial complementarity in the architecture of above-ground and root parts, temporal complementarity [91], or complementarity in the types of resources, hence reducing competition for light, water, and nutrients among plants [92].
On the other hand, facilitation mechanisms occur either directly or indirectly. Direct facilitation describes the physicochemical modifications of the local environment (e.g., increased temperature, enhanced soil moisture, pH modification) induced by one component of the multi-cropping system. It also includes the physical support provided to other plants, which reduces the risk of lodging and hence promotes the growth and development of neighboring plants. Indirect facilitation occurs when the intercropped plants attract external species that provide new ecosystem services, such as soil bacteria or mycorrhizal fungi, that benefit the multi-cropping system [93].
In milpa, complementarity mechanisms are observed both above and below ground among the three crops. For the aerial parts, plant architectural complementarities tend to reduce competition for light (Figure 3). An experimentally validated model of radiation interceptions of maize-bean association simulated a higher radiation use efficiency (RUE) for intercropped beans than sole cropped beans, with no RUE difference between intercropped and sole cropped maize. The model also showed a competitive advantage for the maize compared to bean with higher photosynthetically active radiation (PAR) [94].
Beneficial mechanisms found in milpa. Mechanisms demonstrated in the species composing milpa (maize, common bean, and squash) are written in bold, while those shown in other species belonging to the same families are in italics. The color of the mechanism indicates the species providing the service: gold for maize, green for the bean (and related crops from the same family), orange for the squash. The services provided by all three species of milpa are written in black.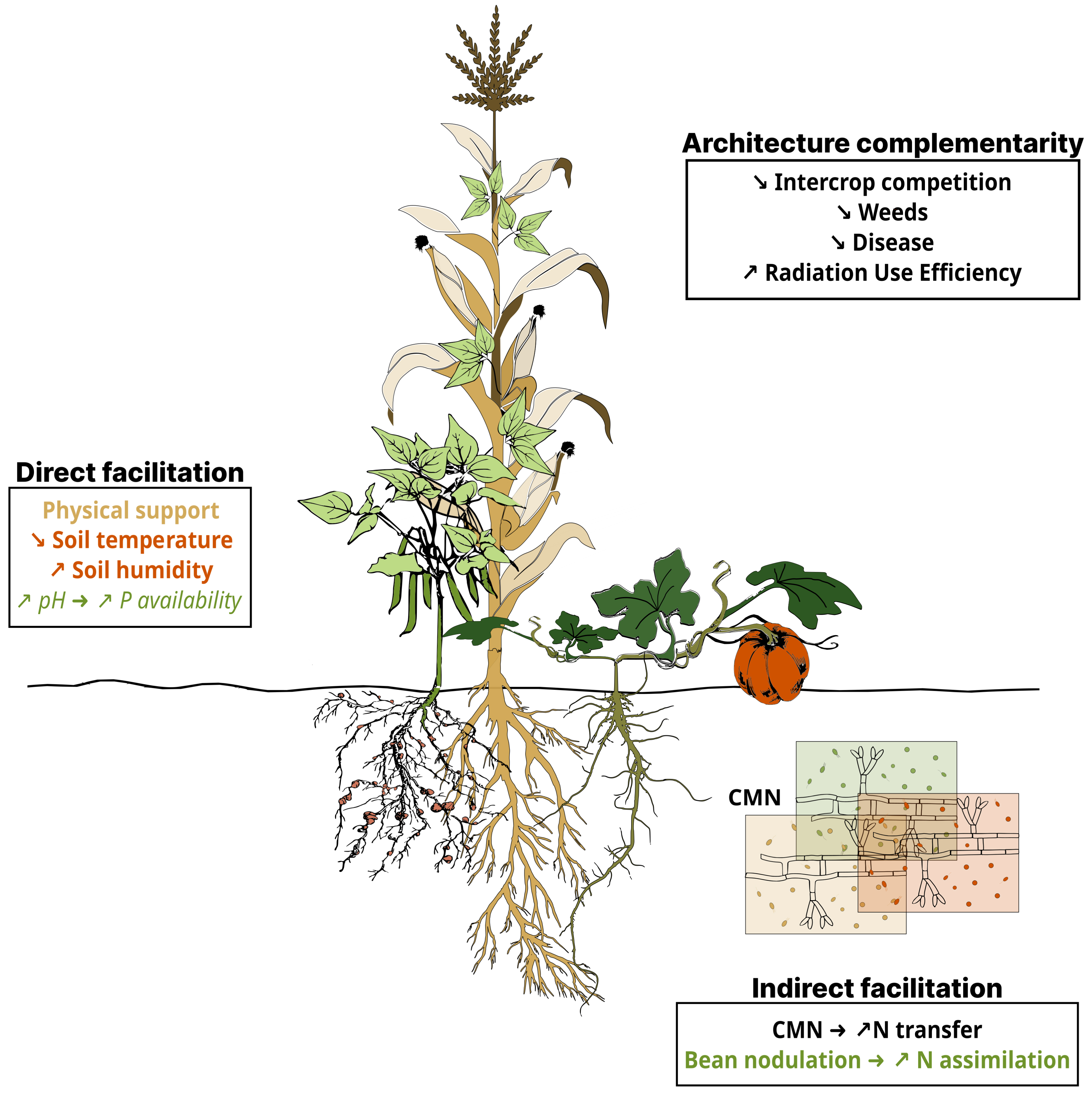
Optimization of space utilization in intercropping systems tends to enhance competitive resistance against weeds. Intercropping maize and bean has been shown to result in lower weed pressure compared to sole-cropping [95]. This difference was attributed to the greater absorption of PAR by the canopy of intercropped maize and beans than by canopies of either crop alone, thereby reducing weed growth [96]. Similarly, intercropping maize with squash reduces weed pressure, primarily due to shading provided by the squash [97].
Above ground, root system complementarities in milpa decrease competition for soil nutrients and water (Figure 3). Hence, simulations of associations of the three crops for nutrient uptake and resource use indicate root architecture complementarity, which results in increased biomass production on nitrogen (N)-deficient soils [98]. Differences in root architecture among these crops indeed reflect a diversity of nutrient foraging strategies, with shallow, more vertical and, deep soil exploration for maize, bean and squash, respectively [89]. There is also evidence that the higher transpiration observed in the associated maize and bean plants compared to maize alone is the result of increased water assimilation, due to higher root proliferation and a more complete exploitation of the water resource in the soil profile [99].
In milpa, direct facilitation primarily occurs through the physical support provided by maize for the growth of bean (Figure 3). Moreover, optimized crop canopies in maize-bean and maize-bean-squash associations reduce soil temperature, increase water availability and electron transport rates compared to maize grown alone [100]. In milpa systems, plants are known to release root exudates that can locally modify rhizosphere properties [101, 102, 103]. Results on maize and faba bean (Vicia faba) intercropping for example, show that the bean’s phosphorus (P) uptake strategy acidifies the rhizospheric soil via root exudate production (Figure 3). This acidification enhances the availability of inorganic P, facilitating uptake by maize [104, 105]. Whether these results apply to maize-common bean intercropping remains to be tested.
Indirect facilitation also occurs in milpa (Figure 3). Most food legumes form nodules to host N-fixing rhizobial bacteria, a process initiated by plant-released flavonoids and rhizobial Nod factors [106]. This signaling pathway, crucial for N fixation in legumes, also supports symbiosis with mycorrhizal fungi [107]. It has been shown that in common bean, this fixation can reach 60% of its total N acquisition [108]. In intercropped maize-bean, the symbiotic N fixation can also benefit maize [109]. In a first step of the culture, when the nodules are not yet developed, the two species compete for soil mineral N. The advantage of the cereal due to its faster root development triggers symbiotic fixation in the legume [110]. Hence a greater proportion of the soil N is available to the cereal in intercropping than in sole-cropped stands, limiting the need for additional fertilization [111]. Additionally, flavonoids found in maize root exudates enhance nodulation in faba bean and improve N uptake [112]. Moreover, the high level of synchrony between N inputs and crop N uptake in the maize-soybean association tends to avoid the build-up of excess N in soils leading to a reduction of nitrate leaching/lixiviation [113]. Whether these results hold for maize-bean intercropping remains to be determined.
Common mycorrhizal networks (CMN) also play a key role in indirect facilitation (Figure 3). It was demonstrated that N assimilation in maize intercropped with bean increased due to N transfer via interplant hyphal connections of arbuscular mycorrhizal fungi [114, 115].
Crop assemblage in milpa also reduces pest and disease pressure through complementarity and facilitation (Figure 3). This involves barrier effects (resistant plants restraining pathogens), dilution effects (lower concentration of potential targets) and compensation effects (poor growth of susceptible plants is compensated by the growth of non-sensitive ones that better utilize resources). In maize-bean intercropping, common bacterial blight and rust diseases decreased by 23% and 51%, respectively, compared with sole-cropping [116]. Likewise, both decrease of the maize pest Rhopalosiphum maidis and reduction of herbivorous beetles were observed in intercropped maize-squash-faba bean [117] compared to maize alone, and similarly in maize-bean-squash [118] compared to sole-cropped plants of each of the three species.
The success of milpa results from ecosystemic services delivered by crop interactions within agricultural systems. These services, extensively studied in natural ecosystems, include net primary production, N mineralization, soil moisture retention, water flow regulation, carbon storage, soil formation, and soil retention [119]. Insights from these studies have inspired agroecology. Conversely, experiments in agronomic contexts, that involve fewer plant species, can serve as valuable models for understanding more complex natural ecosystems.
5. The milpa, a lever for the agroecological transition
Conventional agriculture has significantly boosted food production, but it has also caused severe environmental drawbacks, including increased greenhouse gas emissions, soil degradation, and extensive freshwater withdrawals [120]. A sustainable agricultural model is needed to improve resource efficiency and reduce inputs [121].
Crop mixtures enhance productivity by harnessing beneficial plant–plant interactions instead of relying on chemical inputs. In this context, the well-studied milpa system offers valuable nutritional complementarities among species and serves as an exemplary agroecological model. A study involving 989 Guatemalan small farms practicing traditional and derived forms of milpa showed that one hectare of milpa produces more calories than one hectare of sole cropped maize [87]. Maize primarily provides carbohydrates (68–75%), squashes are rich in lipids (41–49%), and beans offer substantial proteins (19–28%) (Sánchez-Velázquez et al. [122] and references herein). The proteins obtained from milpa can serve as a high-quality substitute for animal proteins, with an amino acid score considering protein digestibility often exceeding FAO/WHO recommendations [122, 123]. Additionally, milpa boasts significant micronutrient content, supplying 11 different vitamins and eight different minerals with interesting complementarities. For example, maize contains a substantial amount of vitamin C, almost absent from bean and pumpkin seeds, while bean is notably rich in vitamin B4 compared with maize and pumpkin seeds, and pumpkin seeds provide greater amounts of vitamins A and B3 [122]. Milpa also contains diverse bioactive compounds such as polyphenols, phytosterols, saponins, fiber, bioactive peptides, and carotenoids, which are linked to various health benefits [124].
To advance the use of milpa in agroecology, there is a crucial need to identify phenotypic traits and their genetic determinants that foster positive plant–plant and plant–microorganism interactions. These traits include those related to aerial and root architectures, to resource acquisition, to root exudation, and to symbiotic relationships with bacteria or mycorrhizal fungi. In maize-bean associations, maize acts a dominant competitor that can affect the overall productivity [125, 126, 127], but can also suffer from lodging due to weight of the bean, highlighting the need to find a balance between low-competitive but robust maize varieties [82].
Recent research explores two primary strategies for selecting multi-cropping: trait-blind and trait-based approaches [128]. Trait-blind selection tests various genotype combinations directly in the field to find the best performers. Trait-based selection focuses on specific traits that enhance desired outcomes, such as efficient N fixation in beans for milpa systems [129, 130]. The latter method requires understanding genetic and phenotypic traits that foster complementary interactions between species. This would help breeders to target beneficial traits increasing yield predictability and sustainability. In both approaches, selection should be conducted in low-input settings to foster synergistic interactions and to allow the improvement of efficient systems based on positive biological interactions rather than chemical inputs [131, 132]. Note that it is possible to optimize the selection schemes by combining the trait-blind and trait-based methods. Initial trait-blind selection can identify promising combinations, which can then be further refined using trait-based methods to enhance specific desirable characteristics [128].
A promising avenue to foster beneficial interactions and improving selection for efficient multi-cropping systems would be to remobilize underutilized genetic pools, such as wild relatives, early domesticated forms, and landraces, that can expand the range of potential plant architectures and functions in multi-cropping systems. Landraces, selected for efficiency in intercropping, likely carry valuable co-adaptive alleles that modern breeding has often neglected [93]. In addition, native maize landraces used in milpa systems possess a more diverse seed-endophytic microbiome with greater antagonistic effect against soil-borne bacteria compared to hybrid varieties [133]. Germplasms of traditional maize, bean, and squash varieties have been preserved both ex situ in American and European GenBanks and in situ in small-scale farms such as in Transylvania. Both in situ and ex situ resources are key to exploring the full range of potential interactions in the milpa systems.
Selecting suitable varieties for multi-cropping systems is complicated by significant genotype x cropping system interactions, as well as practices x genotype x cropping system interactions [134, 126, 82]. Practices include soil management, sowing date, plant density and inputs. Together, they significantly impact productivity and seed quality of intercropping systems. For example, results from alternating rows of maize and common beans indicate that reduced tillage preserved the mycorrhizal network, leading to earlier root colonization and improved nutrition for the beans, which in turn enhanced nitrogen transfer to the maize [115]. Additionally, sowing beans before maize proved beneficial, as it allowed the beans to quickly connect to the mycelial network and reduced competition between the two crops [115]. Regarding inputs, a meta-analysis of maize intercropped with legumes, including common beans, found that high nitrogen inputs reduce the Land Equivalent Ratio (LER) by shifting the balance from complementarity to competition between crops. Under these conditions, the benefits of complementarity diminish, making the competitive interactions more dominant [135]. Note that the natural presence of inoculum, or artificial inoculation of bean seeds, is key to take advantage of the symbiotic nitrogen fixation. Likewise, complementarity in phosphorus uptake also occurs in P-deficient soils, resulting in significant overyielding in maize/faba bean intercropping [104] but also in chickpea/maize [136], white lupin/wheat [137], common bean/wheat [138], and faba bean/wheat [112].
Finally, the large-scale adoption of intercropping is hindered by the lack of suitable mechanization tools [139]. Harvesting and sorting intercropped plants is challenging, as separation depends on species and on combined harvester settings [140]. There are two main possibilities to explore, either converting row-intercropping to strip-intercropping to use existing machinery [115] or developing new machines for row-intercropping. The former may impact beneficial interactions, while the latter will require significant investment. Recently, adjusting harvester settings has enabled successful harvesting and sorting of multi-cropping systems such as wheat-lupin and barley-pea using standard equipment [140], which could be in principle applied to maize-bean intercropping. However, an additional challenge lies in the need for multiple harvest passes in beans.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
NV-B was financed by a doctoral contract from SPS through the Doctoral school “Sciences du Végétal ED567”. This work was funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 862862 (INCREASE Project).
Acknowledgements
We sincerely thank Tudor Ursu and Anamaria Roamn for their insightful discussions on milpa in Transylvania, and Luis E. Eguiarte for his helpful feedback and detailed corrections on the manuscript. We also greatly appreciate Doyle McKey’s valuable comments and edits, which significantly improved the manuscript. We acknowledge the use of OpenAI’s ChatGPT for providing assistance in correcting the text in this manuscript without generating original content.




 CC-BY 4.0
CC-BY 4.0