1 Introduction
In social insects, ‘territoriality’ is generally defined as all the defensive behaviours used against all kinds of intruders in an area centred around the nest entrance. In most ants, territoriality contributes to the defence of spatiotemporally stable food resources and to the trails towards these sources. The range of the area defended can also vary from a restricted zone around the nest entrance 〚1–3〛 to the entire territory (nests and food sources). The first example corresponds to most predatory ant species with individual foraging strategies when faced with unpredictable distributions of prey; the second example is related to absolute territories of arboreal dominant ant species 〚4〛.
The expression of territorial defence also varies according to the behavioural repertoire of the species considered, with intraspecific aggressiveness, often resulting in full attacks that can end with the destruction of one colony by another in Formica spp. Nevertheless, ritualised behaviours in which the participants are not killed also exist both at the intra- and interspecific levels 〚5–8〛.
In predatory ants, it is often difficult to distinguish aggressiveness from predation, as the same behaviours can occur in both cases. Spread-eagling offers a good example of this. Indeed, it is very frequent in ants for several recruited workers to spread-eagle an intruder. If the intruder is overwhelmed, it is very often taken back to the nest to be eaten. In ecologically dominant arboreal species known for their territoriality, spread-eagling is used both against other ants, including conspecifics, and in prey capture, with only very small prey being captured by single workers 〚3, 9–11〛.
It was with the aim of distinguishing territorial aggressiveness from predatory behaviour that we decided to conduct a study on Plectroctena minor, a ponerine ant species equipped with hypertrophied mandibles that can snap 〚12〛 (Fig. 1). The snapping of mandibles results in a sharp blow to intruders, stunning or killing them. Snapping, also noted in soldiers of several termite genera and in the ant genera Mystrium (Ponerinae) and Orectognathus (Myrmicinae), is considered to be firstly used for nest defence 〚13–16〛. Nevertheless, we noted that P. minor workers, rather specialised in millipede capture, sometimes use this behaviour during the predation of alternative prey or large millipedes 〚17–19〛. We therefore compared snapping occurrences when P. minor foraging workers encountered nestmates or alien conspecific workers, workers of sympatric competing ponerine ant species, and different kinds of preys.
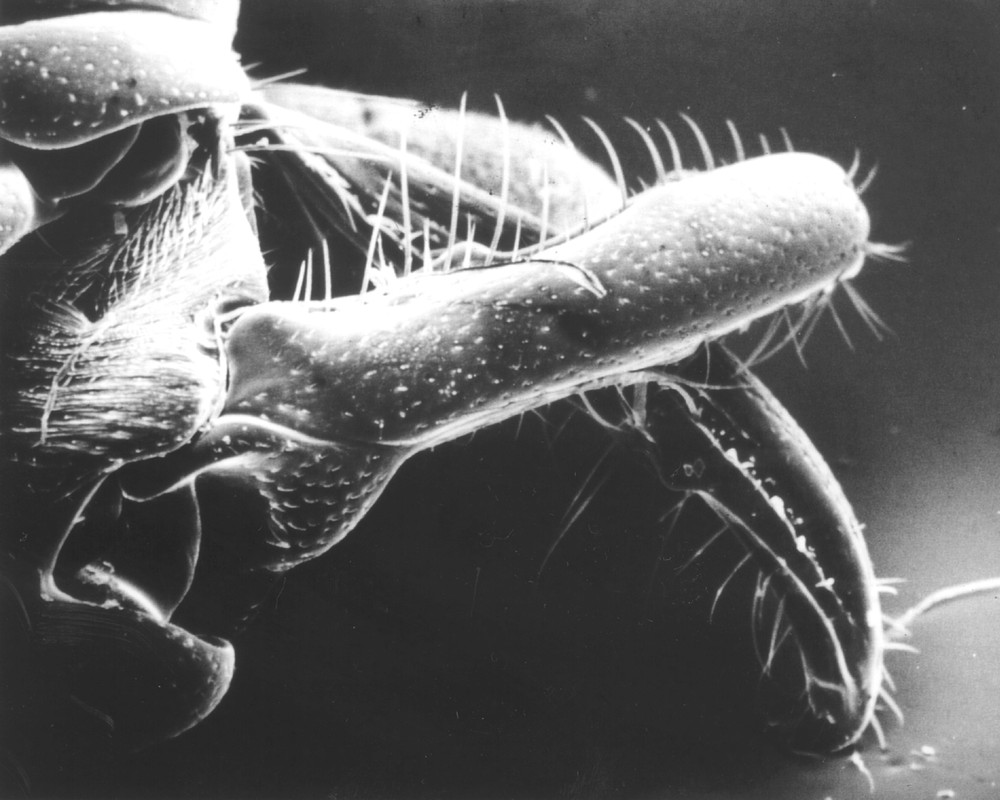
Scanning electron photograph of the mandibles of a Plectroctena minor worker after snapping (mandible length: 3 mm).
2 Materials and methods
We carried out this study in Yaoundé (Cameroon) on five queenright P. minor colonies containing 70 to 100 workers and abundant brood. In the laboratory, the colonies were bred in test tubes (22 × 2.5 cm) supplied with a watering place and opening into hunting arenas (45 × 35 × 5 cm) covered with a plate of glass. These arenas were plastic, without sand or leaf litter, to fully expose the tested items and to standardise the test conditions. Ants marked the nest entrance and the hunting arena with anal spots as their natural foraging area.
In order to study the territorial behaviour of P. minor workers at the intraspecific level, we conducted two series of confrontation tests. In the first series, encounters between P. minor foraging workers occurred in neutral arenas (15 cm in diameter), where two individuals were introduced. In the second series, a worker was introduced into the hunting arena of a colony, and the encounters occurred more than 20 cm away from the nest entrances. Each time, we used homocolonial (the workers were separated for 24 h or during 15 days), then heterocolonial workers.
A study on the reactions of P. minor workers vis-à-vis sympatric, competing ponerine species was conducted by individually introducing workers of these species into the hunting arenas of P. minor colonies. We noted each time if the P. minor workers snapped at the alien or not. In the first series of experiments, we tested Plectroctena gabonensis workers in order to obtain information on the reactions of P. minor workers at the intrageneric level. In the second series of experiments, we tested workers of Leptogenys sp., Pachycondyla analis, Pa. tarsata, Pa. soror, and Pa. pachyderma (all Ponerinae; results pooled). This time, we compared encounters between P. minor workers and test workers taking place in the hunting arenas less than 5 cm vs more than 20 cm from the nest entrances.
We used the same protocol with insects previously known as potential prey of P. minor workers 〚18〛: 5–7 mm long Cubitermes sp. workers, 10–12 mm long oniscoid isopods, 20–25 mm long Grillidae larvae, 25–35 mm long Tenebrionidae larvae, and 30–35 mm long millipedes. We also experimented with large Macrotermes bellicosus soldiers (20–25 mm long) whose role consists in defending termitary chambers by preventing enemies from penetrating into galleries.
For statistical comparisons, we used Fisher’s exact-tests (StatXact-3 software). Appropriate probabilities were adjusted for the number of simultaneous tests, using the sequential Bonferroni procedure 〚20〛.
3 Results
Antennal contact always occurred during encounters, but, depending on the cases, it was followed by a brief antennal palpation (most of the cases) or by the folding back of the antennae when confronted with millipedes and termite soldiers. Snapping never occurred during encounters between homocolonial workers, or in neutral arenas, or when an individual was introduced into the foraging arena of its colony after 24 h or even 15 days of separation (Table 1). On the contrary, snapping was always noted during encounters between heterocolonial individuals. Territorial marks probably play a role in the workers’ behaviour as, in the latter case, it was always the aliens that were snapped at, and they tried to escape before being snapped at in 22 cases out of 35. The strong blow received during snapping sometimes led to the death of the alien worker.
Snapping frequency during encounters between Plectroctena minor workers and others ant species according to the zone of confrontation.
| Neutral arena | Foraging arena | ||
| Homocolonial P. minor workers | 0% (N = 30) | 0% (N = 30) | |
| Heterocolonial P. minor workers | 100% (N = 20) | 100% (N = 35) | |
| P. gabonensis workers | N.D. | 95.6% (N = 23) | |
| < 5cm from nest entrance | > 20 cm from nest entrance | ||
| Sympatric Ponerinae | N.D. | 75% (N = 24) | 33.3% (N = 54) |
A very similar behaviour was noted when P. minor workers snapped at P. gabonensis workers in 22 cases out of 23, independently of the zone of the hunting arenas where they encountered them (Table 1). Otherwise, they snapped at Pachycondyla spp. and Leptogenys workers more frequently when encountered less than 5 cm from the nest entrances than at a distance greater than 20 cm (Fisher’s exact-test: P = 0.0011; Table 1). Note that during these interspecific encounters reciprocal avoidance, and mostly avoidance by alien individuals, was frequent. Nevertheless, fighting was noted in nine cases out of 78 (11.5%) and even resulted once in the death of a P. minor worker.
Except for large Macrotermes soldiers that were snapped at in all cases (Figs. 2 and 3, Table 2), P. minor hunting workers also significantly snapped at their prey more frequently when encountered near the nest entrances than at a distance greater than 20 cm in the hunting arenas (Fig. 3). Otherwise, we noted a gradation in the occurrence of snapping according to prey nature when we compared the reactions of the hunting workers during encounters more than 20 cm from the nest entrances (Fig. 3, Table 2). In all cases, the workers folded their antennae backward when approaching Macrotermes soldiers in order to snap at them. The same behaviour was noted in certain cases for Grillidae.

A Plectroctena minor worker preparing to snap at the head of a Macrotermes bellicosus soldier under laboratory conditions (A). The worker prepares to retrieve the soldier stunned by the snapping (B). Worker length is 17 mm.

Comparison of percentages of prey snapped at according to their nature and their distance from the nest entrance (number of prey tested in brackets). Statistical comparisons of the number of prey snapped at from a distance up to 5 cm and beyond 20 cm from the nest (Fisher’s exact-test).
Statistical comparisons of snapping between prey during encounters occurring at more than 20 cm from the nest entrances (Fisher’s exact-test and sequential Bonferroni procedure).
| Prey nature | Millipedes | Cubitermes workers | Grillidae larvae | Tenebrionidae larvae | Macrotermes large soldiers |
| Isopods | NS | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.001 |
| Millipedes | NS | P < 0.001 | P < 0.001 | P < 0.001 | |
| Cubitermes workers | P < 0.001 | P < 0.001 | P < 0.001 | ||
| Grillidae larvae | NS | P < 0.001 | |||
| Tenebrionidae larvae | P < 0.001 |
4 Discussion
In P. minor intraspecific aggressiveness was illustrated by the systematic occurrence of snapping at alien conspecific workers. This behaviour can be compared to ‘full attacks’ noted in different ant species in the same situation. In these cases, the differences in colony odour (cuticular substances) trigger the aggressive behaviour in the workers, with olfaction being the basis for alien and nestmate recognition 〚5, 7〛. The same was true at the interspecific, intrageneric level when P. minor workers encountered P. gabonensis individuals that were snapped at independently of the zone of encounter in the hunting arenas. On the contrary, other tested ponerine species were snapped at when encountered around the nest entrances rather than elsewhere in the hunting arenas. There are therefore differences in the responses to encounters with alien ants, with greater aggressiveness toward conspecific or congeneric aliens than toward workers of other sympatric ponerine species. These differences might be related to the semi-specialisation of P. minor and other Plectroctena species in the capture of millipedes. The latter, not being the most frequent litter-dwelling arthropods, are essential in the diet of P. minor colonies for worker and queen production 〚17〛. As a result, congeneric individuals are more important competitors than other predatory ant species. Snapping seems then to be indirectly dependent on two principal parameters: the type of encountered individuals and the site of the encounter. The combination of these two factors directly influences the aggressiveness of P. minor workers, and thus snapping.
The initial hypothesis was that snapping is related to territorial aggressiveness. Our results highlight that this behaviour also concerns predation, for which the above-cited assertion remains true. Large Macrotermes soldiers were always snapped at, while prey selected during cafeteria experiments, namely small millipedes, isopods and Cubitermes workers 〚17, 18〛, were the least frequently snapped at. Also, the size of the prey intervenes as large millipedes were snapped at in certain cases, while smaller individuals were not 〚19〛. Snapping frequencies recorded for Grillidae and Tenebrionidae larvae still remain difficult to interpret. As antennal contact always occurred and was followed by palpation or, for dangerous prey, by the folding back of the antennae when confronted with dangerous prey, behaviour previously reported in ponerine ants, we deduced that workers discriminated encountered insects during this contact. One can hypothesise that the nature and the perception of the cuticular substances of these insects trigger aggressiveness and snapping in the ants. As all prey other than termite soldiers were significantly more frequently snapped at when encountered close rather than far from the nest entrances, we can assert that territorial aggressiveness intermingles with predatory behaviour. Although less salient, a similar result was also illustrated by Ectatomma tuberculatum (Ponerinae) hunting workers that stung more frequently small or stunned prey during encounters close rather than far from the nest entrances 〚21〛.
Plectroctena minor workers snapped at all large Macrotermes soldiers, even those encountered far from the nest entrances (this study), while this behaviour decreased to 80% for small soldiers and only 17% for workers 〚18〛. These differences highlight the fact that P. minor workers can discriminate dangerous prey from others of the same species. Termite soldiers are extremely aggressive and can cut a worker into two pieces thanks to their very powerful mandibles. They assume the role of colony defence, plugging galleries, so that termite predators need to eliminate them in order to have access to the rest of the termitary. For this purpose, other ponerine ants bend their gaster in order to direct their devaginated sting toward the termite soldiers, killing them with venom 〚22, 23〛. Snapping seems to be a well-adapted behaviour for stunning or killing termite soldiers, as it does not require the workers to bend their gaster, a behaviour necessitating wider galleries. We found again that ants fold their antennae backward when detecting termite soldiers, this ‘prudence’ behaviour being general in ponerine ants preying on termites (〚22, 23〛 and references cited therein).
According to our results, snapping is then related both to territorial aggressiveness and to prey capture. Plectroctena spp. workers forage under the litter, beneath the bark of rotting logs, or in underground galleries (〚12, 24〛; Dejean, pers. obs.). In this case, the snap-jaw design of the mandibles is advantageous when compared to species equipped with ‘normal’ mandibles that cannot be used to stun prey. The trap-jaws mandibles of Odontomachus and Anochetus permit them to stun prey, but when open are wider than the worker’s body and so poorly adapted to narrow galleries. In conclusion, contrarily to termites for which snapping was selected for nest defence, the evolutionary process that conducted ants to the same adaptation may be different. Indeed, this behaviour could result from an adaptation for preying in galleries, which was secondarily selected for territorial aggressiveness.
Acknowledgements
We are grateful to Dr B. Bolton (Museum of Natural History, London, UK, where voucher specimens were deposited) for the identification of the ants. We are indebted to Andrea Dejean and an anonymous referee for useful comments on the manuscript.
Version abrégée
Chez les insectes sociaux, la territorialité est souvent définie comme correspondant à l’ensemble des comportements liés à la défense d’une aire située autour du nid. Chez les fourmis terricoles, l’aire défendue peut être limitée aux zones situées près du nid ou autour des sources de nourriture, et aux pistes qui y conduisent. Chez certaines espèces arboricoles, elle peut être étendue à un territoire entier. La zone défendue peut l’être contre des colonies de même espèce ou d’espèces différentes, généralement compétitrices, mais aussi contre d’autres animaux. Dans ce dernier cas, il est difficile de faire la distinction entre prédation et territorialité. Nous avons expérimenté sur Plectroctena minor, une fourmi Ponerinae terricole spécialisée dans la capture des iules, mais qui chasse aussi des proies alternatives. Les ouvrières ont des mandibules hypertrophiées pouvant se croiser brutalement en infligeant un choc puissant contre tout ennemi ou proie lorsque, fortement appuyées l’une contre l’autre par contraction des muscles adducteurs, elles se croisent soudainement produisant un claquement bien audible (snapping). Ce comportement, qui ici peut étourdir ou tuer l’adversaire, est utilisé pour la défense des colonies par les soldats de termites Capritermes et Pericapritermes et les ouvrières de fourmis des genres Orectognathus et Mystrium. Nous avons vérifié que ce snapping est bien impliqué dans la défense territoriale chez P. minor lors de tests de confrontation entre ouvrières. En effet, après séparation de 24 h ou de 15 j, les ouvrières provenant d’une même colonie n’effectuent jamais de snapping, alors que ce comportement est toujours utilisé lors des rencontres entre ouvrières de colonies différentes, que ce soit sur une aire neutre ou sur le territoire d’une colonie. Dans ce dernier cas, seules les étrangères, qui essaient généralement de s’échapper, font l’objet de snapping. Ce comportement, qui se retrouve lors de confrontations avec d’autres Ponerinae sympatriques, a donc aussi une valeur interspécifique. Sur les aires de chasse des colonies, le comportement des ouvrières varie en fonction de la nature de la proie et du point de rencontre avec les proies. Le snapping est peu fréquent vis-à-vis des iules de petite taille et des isopodes, intermédiaire pour les ouvriers de termite Cubitermes, fréquent pour les larves de grillons et de Tenebrionidae et systématique vis-à-vis des grands soldats du termite Macrotermes bellicosus. En dehors de ces derniers, les proies testées font plus souvent l’objet de snapping quand elles sont rencontrées sur la zone de l’aire de chasse située à moins de 5 cm de l’entrée du nid que sur des zones plus éloignées (plus de 20 cm de l’entrée du nid). Il y a donc une sorte de mixité entre les comportements prédateur et territorial. Dans la prédation, le snapping a une forte valeur adaptative pour la chasse dans les galeries, car il permet aux ouvrières de se déplacer puis d’attaquer des proies tout en gardant les mandibules fermées. Ces ouvrières peuvent donc utiliser des galeries d’un diamètre à peine supérieur à celui de leur corps. Ce n’est pas le cas chez les autres fourmis qui ne pratiquent pas le snapping, en particulier les Odontomachus et les Anochetus, qui chassent avec leurs mandibules hypertrophiées ouvertes à 180°.



Vous devez vous connecter pour continuer.
S'authentifier