1 Introduction
Sex-ratio theory predicting deviations from equal maternal investment into sons and daughters has been the most convincing demonstration of the validity of the resource allocation theory [1]. Data on fig-pollinating wasps has been central in this demonstration [2–4]. These wasps breed under conditions of local mate competition (LMC) [5]: broods are laid in patches (figs), and all matings occur within a patch among offspring before mated females disperse. As predicted by theory, colonising females lay higher proportions of males as the number of females colonising the same patch increases [6]. A complementary body of theory predicts that, under LMC, a single female ovipositing on a site should lay a higher proportion of males when her clutch is small [7].
The rationale behind sex-ratio adjustment to clutch size is quite simple. When single, founding females are selected to maximise the number of mated daughters that will leave the patch, and therefore selection favours females that lay just enough sons to inseminate all their daughters [5]. For instance, if one son were capable of mating 20 daughters, the ideal for the foundress would be to produce exactly one son for every 20 daughters. However, if she lays one male too few, she loses 20 mated daughters, while if she lays one male too many, she loses only one mated daughter. Thus, the cost of sex-ratio variance is highly asymmetric. Sex-ratio variance should therefore result in selection of less female biased sex ratios. This variance depends on the brood size. If egg sex determinism is binomial, and if X is the proportion of male eggs, the variance in total number of males will be and sex-ratio (number of males divided by clutch size) variance will be . Hence, sex-ratio variance automatically decreases with brood size. Therefore, within species, founding females laying larger broods should be selected to lay a lower proportion of insurance males, and so sex ratios should decrease with the brood size. The effect of a similar property, the necessity of producing at least one male, has been modelled and tested for some species of parasitoid wasps and mites producing small clutches, at most 30, so that a single male was sufficient to mate all daughters [8–10]. Another prediction of this category of models is that, because of the cost of laying insurance males, females should be selected to lay more precise sex ratios than binomial when brood size is small [11]. The associated literature is, however, confusing, because the term sex-ratio variance is often used improperly to mean (observed sex-ratio variance)/(expected binomial sex-ratio variance), instead of, simply, observed sex-ratio variance.
In order to test the effect of clutch size on sex ratio within species with large brood sizes (up to 400 offspring), we collected sex-ratio and clutch size data in single-foundress figs for six species of fig-pollinating wasps belonging to five genera. We show the brood size effect to be strong. Further, when several females compete, individual clutch size is reduced [12]. As a result, the proportion of males increases, and this could provide a mechanistic explanation of sex-ratio response to numbers of colonizing females. Therefore, sex ratio data on fig wasps need to be reassessed to determine whether females count other foundresses, as is generally accepted, or whether they simply count the number of eggs that they lay.
2 Materials and methods
2.1 Study species
Data were collected on Pegoscapus tristani pollinating Ficus pertusa (section Americana) in Costa Rica, Courtella gabonensis pollinating Ficus ottoniifolia ottoniifolia (section Galoglychia subsection Caulocarpae) and Alfonsiella fimbriata pollinating Ficus natalensis leprieuri (section Galoglychia subsection Chlamydodorae) in Gabon, Alfonsiella sp. indet. pollinating F. craterostoma (section Galoglychia subsection Chlamydodorae) and Allotriozoon heterandromorphum pollinating F. lutea (section Galoglychia subsection Galoglychia) in South Africa, and Liporrhopalum tentacularis pollinating F. montana (section Sycidium) from Indonesia in a glasshouse population in Leeds (UK) [13]. Species names follow Wiebes [14–16].
2.2 Data collection
Figs were enclosed individually in vials, and wasps were allowed to emerge. All wasps emerging from galls as well as wasps still enclosed in galls were counted under a dissecting microscope. The protocol for establishing foundress number varied according to the biology of the species. In L. tentacularis, females frequently escaped from the figs in which they had oviposited, so those data were obtained through controlled introductions of single foundresses into figs [13]. For the remainder of the species, naturally visited figs were used. For Courtella gabonensis and Alfonsiella fimbriata, the remains of dead foundresses could readily be counted once offspring had emerged from the fig, so that single foundress status was established while counting the offspring. For Alfonsiella sp. indet. and Allotriozoon heterandromorphum, foundresses had to be counted by splitting open the fig just before wasp emergence. For Pegoscapus tristani, counting foundresses at fig maturity was not feasible, so that only crops in which dissections of large samples of newly pollinated figs indicated that they had been uniformly visited by a single wasp were used.
2.3 Statistical analyses
Statistical analyses were carried out using GLIM. When sample sizes were sufficiently large and several crops had been collected, data were analysed separately by crop (two crops, A. fimbriata; four crops, P. tristani). Within-species correlation between sex ratio and clutch size in single-foundress figs was established using binomial variance models (logistic regression in Generalized Linear Models) [17]. Sex ratio was regressed directly against clutch size, but also against log transformed and log–log transformed clutch size to allow for different curvatures (non-linearity), and the model giving the smallest residual deviance was retained. As the ratio of residual deviance and degrees of freedom was rarely close to one, F tests were used. Ratios higher than one indicate higher than binomial variance, or an inappropriate model; ratios lower than 1 indicate lower than binomial variance. This ratio is called the Heterogeneity Factor (HF).
To rule out the possibility that the sex-ratio response to clutch size resulted from foundresses producing a constant number of males [17], the presence of a correlation between the number of males and the number of females in a clutch was tested with a generalised linear model with Poisson variance (data on male numbers are counts).
The interspecific analysis was done on 16 species through standard multiple regression linear model analysis, using the data in West and Herre [11], as this is the sole dataset providing values averaged over large numbers of crops and figs. Sex-ratio variance in single foundress figs was calculated from HF (heterogeneity factor) and GV (Green variance) [7], which are two estimates of the ratio of observed variance in numbers of males divided by expected variance in this number if the distribution were binomial. As the results were identical, we only give results for calculations using HF. Calculated sex-ratio variance is , with X being the average sex ratio of a species and clutch size the average clutch size of a species. It should be noted that West and Herre [11] improperly used the term sex-ratio variance for GV and HF. In our model, we explain sex ratio and sex-ratio variance data, using proportion of single foundress figs within a species and average clutch size as explanatory variable. Type-1 and type-3 models gave the same results. The response of single-foundress sex ratio to average number of foundresses in the species was tested by West and Herre [11], but they did not examine the confounding effect of clutch size. The effect of clutch size had been analysed by Herre et al. [6], but only on a subset of the data, including species usually ovipositing alone.
3 Results
Binomial variance models are very sensitive to strongly deviant data points. This was the case for the single crop that yielded a non-significant correlation between sex ratio and the clutch size (, one crop of Pegoscapus tristani), in which a female (probably unmated, as unmated females give birth to all-male parthenogenetic broods [18,19]) produced a large all-male brood. When that data point was removed, the correlation became highly significant () and explained most of the deviance. Similarly, in Alfonsiella sp. indet., some unusually high sex ratios in relatively large clutches could have to be explained by sperm limitation. Such aberrant points (which did not alter the global results on sex ratio) have a strong influence on the significance of the correlation between male number and female number, and could explain the lack of positive correlation in some species.
Within all species investigated, there was substantial sex-ratio variance. In only three species – Courtella gabonensis, Allotriozoon heterandromorphum and Liporrhopalum tentacularis – was clutch sex-ratio variance somewhat lower than expected under a binomial sex determination mode. As predicted, sex ratio decreased with increasing clutch size. The result held true for species with very large clutches (Fig. 1, Table 1). In several species and crops, male number increased with female number (Table 1): founding females do not begin by first laying a constant number of male eggs and then subsequently laying female eggs.
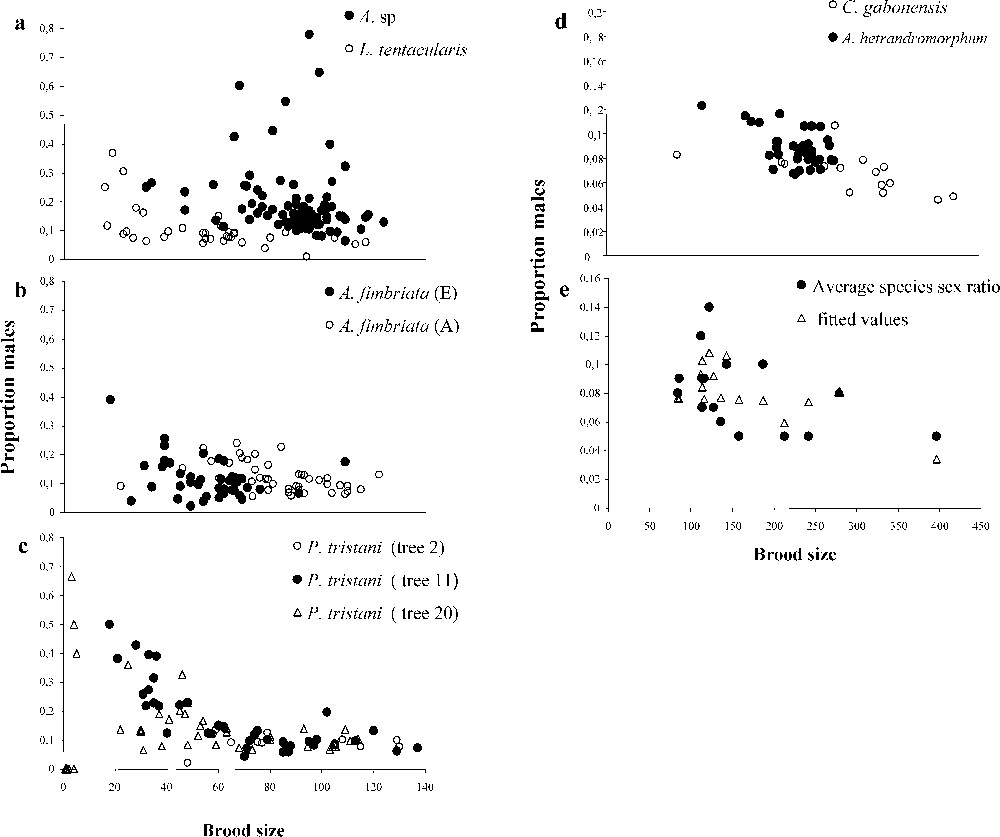
The negative correlation between clutch size and the sex ratio in single foundress figs: (a)–(d) within species data; (e) among species variation (data after West and Herre [11]). Fitted values are based on a multiple regression analysis taking into account the factors clutch size and average numbers of foundresses per fig in the species.
Summary of statistics for within-species analysis
| Species/crop | Significance of correlation between clutch size and sex ratio | Best model | Dispersion | Significance of correlation between number of males and number of females |
| Pegoscapus tristani 501–20 | <0.05 | linear | 1.6 | <10−2 |
| P. tristani 111–11 | 0.06 | log–log | 13 | NS |
| P. tristani (111–11 without aberrant point) | <10−6 | log | 1.9 | NS |
| P. tristani 19 | <10−3 | log | 0.40 | <10−3 |
| P. tristani 503–20 | <10−2 | linear | 0.84 | <10−3 |
| Alf. fimbriata 1 | <10−3 | linear | 1.26 | NS |
| Alf. fimbriata 2 | 0.03 | log–log | 1.56 | NS |
| Alf. sp. in det. | 0.05 | linear | 7.4 | NS |
| Courtella | 0.01 | linear | 0.77 | 0.05 |
| Allotriozoon | <10−3 | linear | 0.41 | <10−3 |
| Liporrhopalum | <10−4 | log | 0.91 | 0.09 |
We also re-examined the negative correlation between clutch size, sex ratio and sex-ratio variance in an interspecific comparison of mean values using the data on 16 other fig-wasp species from West and Herre [11]. Two variables jointly explained the observed sex ratio values in single-foundress figs: average within-species proportion of females ovipositing alone (, negative correlation), but also average clutch size (, negative correlation) (proportion females single). Similarly, sex-ratio variance in single-foundress figs was explained by the proportion of single-foundress figs () and clutch size (, negative correlation). The negative correlation between clutch size and sex-ratio variance apparently contradicts West and Herre [11]. However, note that they were in fact investigating sex-ratio variance divided by expected binomial variance, rather than variance as we have done here.
4 Discussion
We have shown here that in fig pollinating wasps, sex-ratio variance decreases with increasing clutch size, and this is associated with the predicted sex ratio decrease with clutch size, both within and among species. The decrease in sex ratio is still observed for species characterized by large clutch sizes, showing that it is not just the result of selection to produce at least one male. Thus, the result previously observed in organisms with very small clutches, in which one male is sufficient to mate all females [8,9], holds true for species producing clutches about 20 times larger in which one male cannot mate all females. This generalised response to clutch size is in qualitative agreement with theory [7]. It would be much more difficult to assess whether there is also a quantitative fit. Indeed, fig-pollinating wasps are subject to a series of mortality risks such as kleptoparasites, which may induce uncontrolled variance in mortality rates [20]. The high variance compared to binomial variance in the proportion of males is somewhat unexpected.
It has been proposed for some species of parasitoid wasps that the sex-ratio response to number of foundresses on a patch is in fact a response to reduction of individual brood size [12]. Could this be the case for fig pollinating wasps? In fig pollinating wasps, total brood size increases less than linearly with number of foundresses [13,21]. Hence, the sex ratio response to reduction in clutch size per foundress should automatically result in a positive correlation between number of foundresses and the sex ratio. There could be no need for any direct response of foundresses to the presence of other females to lead to ESS sex ratios. Consistent with this interpretation, in Blastophaga nipponica, when two females were introduced sequentially, the second female produced fewer offspring and higher sex ratios [22], but lack of curvature optimisation in the model used for data analysis precludes precise hypothesis testing. In agreement, in Ceratosolen capensis, when a second female was introduced, she laid the same clutch size as the first one and showed no sex-ratio increase [23]. Hence, the generally accepted idea that fig pollinating wasps ‘count’ other foundresses to adjust the sex ratio of their clutches [2,3,24] needs to be reassessed.
Sex ratios have also been proposed and used as an indirect measure of the number of foundresses per patch, or more precisely, of local inbreeding in diverse organisms [25,26]. This approach has, in turn, been used to demonstrate that agonistic interactions may be independent of kin selection [25], an often-quoted conclusion. The results obtained here show that when clutch size varies, such an estimate of local relatedness may be highly misleading within and among species.
In conclusion, while fig-pollinating wasps have long been thought to have provided a convincing demonstration that females ‘count’ other foundresses to adjust the sex ratios of their broods [2,3,24], this adjustment could result from the simple sex-ratio response to clutch size that we demonstrated here.
Acknowledgments
Work by J.C.M. was mainly carried out while at Centre for Biodiversity and Conservation, School of Biology, University of Leeds, Leeds LS2 9JT, UK.


