1 Introduction
Treefrogs have always called for particular interest among scientist and amateurs. This is due to their particular vivid and pleasant coloration, interesting breeding behaviour and particular locomotion [1]. Recently scientists showed particular interest in study of the phylogenetic relationship of the Rhacophorinae or Rhacophoridae, considered as subfamily or family according to the authors, their taxonomic position in the family Ranidae, and the intragroup relationships. Despite their relative popularity, the group and species involved, in particular the members of the genus Rhacophorus, are still poorly studied and new species are regularly described [3–8].
Rhacophorus reinwardtii (Schlegel, 1840) is one of the first species of oriental amphibians discovered by Kuhl and van Hasselt [9] when they explored Java, and one of the few species of amphibians known to general zoologists [10]. It is a member of the genus Rhacophorus that currently includes about 60 species [11]. The monophyly of this genus seems to be confirmed [12,13]. By recognizing 10 species groups, Dubois [14] tried to organize the poor knowledge we have on a genus that has been revised as a whole in the thirties of the 20th century for the last time [2] and that is poorly represented in collections despite the popularity of some species.
Rhacophorus reinwardtii from Java is a large-sized frog with complete webbing on the hand and dermal appendages on various parts of the body. It shares this characters with R. nigropalmatus from Borneo, which can be distinguished easily by more rough skin and webbing of hand reaching disks of all fingers. Specimens from the northern mountain region (including northern Thailand, northern Laos, northern Vietnam and southern China) [15] have been reported as Rhacophorus nigropalmatus by Smith [16], Van Kampen [17], Smith [18], Ahl [19], Bourret [20], Taylor [21], Ohler et al. [5], but were mentioned in most recent publications as R. reinwardtii by Liu and Hu [22], Yang [23], Manthey and Grossmann [24], Fei [25], Inger et al. [4], Orlov et al. [6], Ziegler [26], Chan-ard [27].
When comparing specimens assigned to Rhacophorus reinwardtii-nigropalmatus from different populations of South-East Asia, gross morphological differences could be found. We tried to confirm this by detailed morphometrical analysis that has shown its efficiency in other cases of species level differentiation, and we analysed DNA sequences obtained from specimens of three different populations. The results are presented in this paper and they lead us to describe a new sibling species of Rhacophorus.
2 Material and methods
2.1 Description and morphometrics
The comparisons and the methodology of description follow Ohler et al. [5,28] and Veith et al. [29] (see Appendix A for list of specimens studied).
The following abbreviations are used for measurements: SVL, snout vent length. Head, HW, head width; HL, head length (from back of mandible to tip of snout); MN, distance from back of mandible to nostril; MFE, distance from back of mandible to front of eye; MBE, distance from back of mandible to back of eye; IFE, distance between front of eyes; IBE, distance between back of eyes; IN, internarial space; EN, distance from front of eye to nostril; EL, eye length; SN, distance from nostril to tip of snout; SL, distance from front of eye to tip of snout; TYD, greatest tympanum diameter; TYE, distance from tympanum to back of eye; IUE, minimum distance between upper eyelids; UEW, maximum width of inter upper eyelid. Forearm, HAL, hand length (from base of outer palmar tubercle to tip of toe); FLL, forelimb length (from elbow to base of outer tubercle); TFL, third finger length (from base of first subarticular tubercle); fd1–fd4, width of pads of finger 1 to 4; fw1–fw4, width of fingers 1 to 4; Hindlimb, FL, femur length (from vent to knee); TL, tibia length; FOL, foot length (from base of inner metatarsal tubercle to tip of toe); FTL, fourth toe length (from base of first subarticular tubercle to tip of toe); td1–td5, width of pads of toes 1 to 5; tw1 to tw5, width of toes 1 to 5; IMT, length of inner metatarsal tubercle; ITL, inner toe length. Webbing, MTTF, distance from distal edge of metatarsal tubercle to maximum incurvation of web between third and fourth toe; TFTF, distance from maximum incurvation of web between third and fourth toe to tip of fourth toe; MTFF, distance from distal edge of metatarsal tubercle to maximum incurvation of web between fourth and fifth toe; FFTF, distance from maximum incurvation of web between fourth and fifth toe to tip of fourth toe.
Museum abbreviations: BMNH, Natural History Museum, London, United Kingdom; MNHN, ‘Muséum national d'histoire naturelle’, Paris, France; RMNH, Naturalis, ‘Nationaal Natuurhistorisch Museum’, Leiden, The Netherlands.
Other abbreviations: Ad., adult; Juv., juvenile; Ma, male; Fem.: female; pm, per thousand.
2.2 Protocol for molecular studies
We sequenced 1323 pairs of bases, corresponding to portions of the mitochondrial genes 12S and 16S.
Tissue samples (muscle or liver; either fresh or preserved in 98% ethanol) were available from 12 ranoid species (11 Rhacophorinae and 1 Mantellinae). DNA was extracted using the protocol followed by Delorme [30]. We amplified two fragments of 12S rRNA gene using primers of Delorme et al. [31], except for Polypedates leucomystax, for which we used a particular primer for the light chain [13], and for Rhacophorus sp. nov., for which we used the primer pair of Kocher et al. [32] (L1091 and H1478).
We used the primers of Palumbi et al. [33] to sequence one fragment of the 16S rRNA gene. We followed the PCR conditions as given in Vences et al. [34] and the PCR products were purified and sequenced using automatic sequencers (CEQ 2000 Beckmann). The sequences (see Appendix A for Genbank accession numbers) were aligned using the program Se–Al [35], and by taking into consideration the secondary structure of molecules [36,37]. We also included data available in Genbank for these genes from three species: Rhacophorus sp. nov., Chirixalus doriae and Philautus aurifasciatus.
To assess whether the different gene fragments could be submitted to combined analysis, we tested all possible combinations using the partition homogeneity test (parsimony method of Farris et al. [38], as implemented in PAUP*, version 4b8 [39]). Prior to phylogenetic reconstruction, we explored which substitution model fits the best our sequenced data using the program MODELTEST [40].
Phylogenetic analyses were carried out using PAUP*. We calculated maximum-parsimony (MP) and maximum-likelihood (ML) trees. In the MP analyses, we conducted heuristic searches with initial trees obtained by branch swapping using the TBR (tree bisection-reconnection) routine implemented in PAUP*. Ten random addition sequence replicates were carried out. The ML trees were obtained using heuristic searches, using the substitution model proposed by MODELTEST.
Following Hedges [41], 2000 bootstrap replicates [42] were run in the MP analysis. We used Bayesian inference in the program MrBayes 2.01 [43] for ML analysis, for which we run four simultaneous Metropolis-coupled Monte Carlo Markov chains for 500 000 generations, sampling a tree every ten generations. The initial set of generations needed before convergence on stable likelihood values (‘burnin’) was set at 50 000 (10%) based on empirical evaluation.
3 Results
3.1 Colour pattern
When comparing Rhacophorus reinwardtii from Java with specimens from northern Vietnam and Laos, several colour pattern differences can be observed (Table 1). Though dorsal colour is similar, usually green in life and lavender when stored in alcohol, white spots of moderate size can be regularly seen in frogs from Vietnam and Laos, but have not been observed in frogs from Java. In all specimens a black spot can be observed in the armpit (Fig. 1). It shows sexual dimorphism and is less distinct in females. The posterior part of the thigh is always of uniform colour, light greyish brown of light bluish brown, in Javanese frogs, but in the Laos and Vietnam specimens a brown network on creamy white ground can be more or less distinct. The most peculiar colour difference is in the coloration of the webbing, which is entirely black with longitudinal whitish lines in Rhacophorus reinwardtii from Java (Fig. 2a). In the specimens from the northern area, the black zone of the webbing never goes as far as to the border of the web, but a rather broad creamy white band can be observed, which is distinct from the longitudinal whitish lines present in Rhacophorus reinwardtii from Java (Fig. 2b). In life the black zones are similar in coloration, but the creamy white band in Laos and Vietnam frogs is orange yellow, whereas the longitudinal white lines of Javanese frogs are bluish white. There is some variation in the extension of the black spots, but not in the presence of longitudinal white lines and in the presence of creamy white (orange) border. The syntypes of Hyla reinwardtii Schlegel, 1840 show very distinctly the longitudinal white lines on an all black web.
Morphological comparison of three species of Rhacophorus: R. kio, R. nigropalmatus and R. reinwardtii
| Character | R. kio | R. nigropalmatus | R. reinwardtii |
| Size male | 58.0–79.1 | 82.9–87.2 | 41.1–52.5 |
| Size female | – | 85.2–97.7 | 60.5–79.6 |
| Coloration | |||
| Dorsum | lavender | lavender | lavender |
| White dots on dorsum | present | present | absent |
| White spots on dorsum | none or few | few | absent |
| Flanks | lavender above, dark grey network with light spots, sometimes indistinct | lavender wish brown network ventrally | light brown with whitish spots corresponding to skin glands |
| Spot at armpit | inky black | absent | black, less distinct in females |
| Thigh | creamy white with indistinct brown markings forming in some specimens network | light brown or lilac, sometimes with indistinct brownish network | light greyish brown or light bluish brown |
| Web | inky black at base with creamy white border | blackish with longitudinal whitish lines, more dense distally | entirely black with longitudinal whitish lines |
| Dorsal skin | finely shagreened | shagreened | smooth |
| Webbing | |||
| Hand | -1-0-0-0-0 | Complete | 2-1-0-0-0-0 |
| Foot | Complete | Complete | Complete |
| Flap of skin | |||
| forearm | well developed | well developed | well developed |
| Tarsus | distinct | distinct | distinct |
| Heel | spine like | rounded | spine like |
| Vent | distinct double-lobed | distinct | distinct double-lobed |

Rhacophorus kio sp. nov., holotype MNHN 2004.0411, adult male, SVL 70.5 mm. Life photography by Thomas Calame, ‘Association Alcide-d'Orbigny’, Clermont-Ferrand, France.
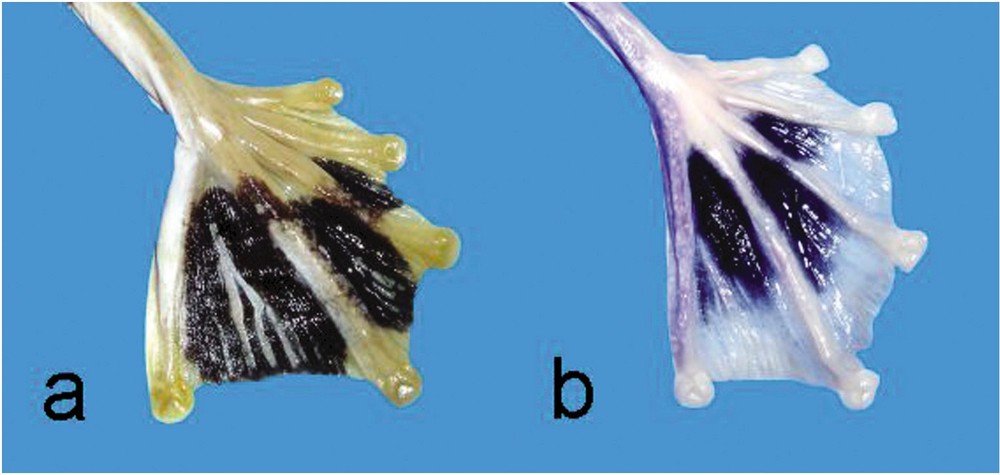
Colour pattern of webbing of (a) Rhacophorus reinwardtii, MNHN 1912.0033, adult female, Java, and (b) Rhacophorus kio n. sp., MNHN 2004.0411, holotype, adult male, Laos.
3.2 Morphometrical study
In a preliminary study on the morphological differentiation only 4 measurements were taken on specimens of Rhacophorus reinwardtii. When comparing the samples of adult males of R. reinwardtii to the frogs from northern Laos and northern Vietnam (Table 2), they show significant differences for snout-vent length and head width, R. reinwardtii males being significantly smaller than R. sp. nov. and the head of the former being significantly larger than the head of the new species. This result is particularly important for the interpretation of the comparison of the syntypes and topotypes of R. reinwardtii with the specimens of the new species, as for the first we only had adult females available and for the new species only males are known. We have to be sure not to consider sexual dimorphism as characters of the new taxon.
Morphometric data for adult specimens of Rhacophorus kio n. sp., Rhacophorus reinwardtii and Rhacophorus nigropalmatus and pairwise comparison of adult male specimens with Mann–Whittney U-test
| R. kio | R. reinwardtii | R. nigropalmatus | K–R | K–N | |||
| Males | Males | Females | Males | Females | |||
| N=10 | N=5 | N=10 | N=2 | N=1 | |||
| SVL | 67.1±6.10 | 49.3±4.67 | 67.5±6.64 | 97.7 | U=0.0 | U=0.0 | |
| 58.0–79.1 | 41.6–52.5 | 55.4–79.6 | 82.9–87.2 | p=0.001*** | p=0.030* | ||
| HW/SVL | 315±13.1 | 360±14.3 | 361±14.7 | 346 | U=0.0 | U=0.0 | |
| 298–335 | 344–382 ± | 338–386 | 336–337 | p=0.001*** | p=0.030* | ||
| HL/SVL | 326±15.9 | 342±13.8 | 341±12.4 | 338 | U=12.0 | U=9.0 | |
| 293–346 | 331–361 | 324–368 | 290–346 | p=0.129 n.s. | p=0.909 n.s. | ||
| TL/SVL | 478±15.6 | 491±14.9 | 504±19.1 | 556 | U=17.0 | U=0.0 | |
| 450–493 | 478–514 | 473–527 | 550–583 | p=0.371 n.s. | p=0.030* |
Comparing adult specimens of R. sp. nov. to the syntypes and topotypes of R. reinwardtii discloses numerous significantly different measurements (Table 3). They confirm that R. sp. nov. has a narrower head (Fig. 3) than R. reinwardtii (smaller HW, IFE, IBE, IN) and has a shorter head (smaller HL, MN, MFE, MBE). Both are differentiated in various measurements on fore legs and hind legs. In particular, Rhacophorus sp. nov. has relatively smaller toe pads then R. reinwardtii.
Morphometric comparison of adult specimens of Rhacophorus reinwardtii and Rhacophorus kio n. sp.
| Measurement | Rhacophorus reinwardtii | Rhacophorus kio | Mann–Whittney U test |
| SVL | 66.5±6.53 | 67.1±6.10 | U=23.5 |
| 55.4–71.5 | 58.0–79.1 | p=0.859 n.s. | |
| HW/SVL | 371±7.279 | 315±13.067 | U=0.0 |
| 362–381 | 298–335 | p=0.001*** | |
| HL/SVL | 356±17.908 | 326±15.876 | U=4.0 |
| 338–381 | 293–346 | p=0.008** | |
| MN/SVL | 290±8.346 | 265±30.841 | U=7.0 |
| 280–300 | 183–293 | p=0.028* | |
| MFE/SVL | 223±7.799 | 204.34±10.208 | U=3.5 |
| 213–233 | 189–227 | p=0.005** | |
| MBE/SVL | 125±8.174 | 114±9.374 | U=6.0 |
| 116–138 | 101–135 | p=0.019** | |
| IFE/SVL | 222±10.416 | 198±10.836 | U=2.0 |
| 208–233 | 184–223 | p=0.003** | |
| IBE/SVL | 328±8.489 | 292±10.998 | U=0.0 |
| 315–336 | 275–313 | p=0.001*** | |
| FLL/SVL | 212±11.896 | 192±7.957 | U=6.0 |
| 191–221 | 177–201 | p=0.019** | |
| HAL/SVL | 324±16.688 | 298±20.788 | U=8.0 |
| 303–343 | 264–328 | p=0.040* | |
| TFL/SVL | 196±6.055 | 174±16.284 | U=0.0 |
| 188–202 | 140–186 | p=0.001*** | |
| TL/SVL | 507±21.161 | 478±15.633 | U=8.0 |
| 481–527 | 450–493 | p=0.040* | |
| FOL/SVL | 502±19.656 | 473±18.109 | U=7.5 |
| 480–530 | 440–490 | p=0.028** | |
| FTL/SVL | 271±13.833 | 256±14.581 | U=14.0 |
| 255–288 | 234–277 | p=0.206 n.s. | |
| IN/SVL | 108±6.837 | 94±6.219 | U=2.0 |
| 98–116 | 84–107 | p=0.003** | |
| EN/SVL | 85±5.149 | 88±6.684 | U=17.0 |
| 79–91 | 79–97 | p=0.371 n.s. | |
| EL/SVL | 121±13.428 | 119±9.390 | U=20.0 |
| 103–133 | 105–130 | p=0.594 n.s. | |
| TYD/SVL | 75±4.836 | 69±4.648 | U=10.0 |
| 71–83 | 63–77 | p=0.075 n.s. | |
| TYE/SVL | 27±2.813 | 22±6.604 | U=14.0 |
| 24–31 | 8–29 | p=0.206 n.s. | |
| IMT/SVL | 39±3.386 | 39±3.749 | U=22.5 |
| 33–42 | 35–50 | p=0.768 n.s. | |
| ITL/SVL | 158±7.206 | 143±9.727 | U=5.0 |
| 149–167 | 128–158 | p=0.013** | |
| MTTF/SVL | 357±12.329 | 336±10.097 | U=3.0 |
| 345–374 | 318–353 | p=0.005** | |
| MTFF/SVL | 376±24.663 | 361±15.988 | U=16.0 |
| 353–418 | 342–389 | p=0.310 n.s. | |
| FTTF/SVL | 146±19.558 | 130±15.769 | U=11.0 |
| 117–171 | 101–152 | p=0.099 n.s. | |
| FFTF/SVL | 175±30.310 | 161±34.520 | U=15.0 |
| 132–206 | 135–253 | p=0.254 n.s. | |
| WTF/SVL | 182±8.631 | 180±12.837 | U=21.0 |
| 168–190 | 163–202 | p=0.679 n.s. | |
| WFF/SVL | 191±10.143 | 186±13.842 | U=17.0 |
| 177–205 | 169–219 | p=0.371 n.s. | |
| WI/SVL | 162±6.302 | 152±8.521 | U=8.0 |
| 155–171 | 135–165 | p=0.040* | |
| WII/SVL | 152±11.897 | 151±13.840 | U=24.0 |
| 142–171 | 130–174 | p=0.953 n.s. | |
| FL/SVL | 487±12.555 | 454±20.692 | U=3.0 |
| 467–500 | 423–480 | p=0.005** | |
| SL/SVL | 186±3.631 | 177±9.696 | U=11.0 |
| 182–191 | 162–190 | p=0.099 n.s. | |
| TW/SVL | 111±9.937 | 84±7.848 | U=1.0 |
| 96–120 | 71–97 | p=0.001*** | |
| TFOL/SVL | 743±19.510 | 682±26.800 | U=0.0 |
| 723–771 | 635–714 | p=0.001*** | |
| NS/SVL | 89±11.034 | 81±6.626 | U=12.0 |
| 76–105 | 70–89 | p=0.129 n.s. | |
| IUE/SVL | 115±8.672 | 109±8.253 | U=16.0 |
| 103–124 | 96–126 | p=0.310 n.s. | |
| UEW/SVL | 93±10.422 | 92±4.911 | U=18.0 |
| 75–102 | 85–99 | p=0.440 n.s. | |
| VAI/SVL | 55±3.140 | 50±2.154 | U=0.0 |
| 53–61 | 47–53 | p=0.001*** | |
| VAIII/SVL | 86±7.278 | 75±5.455 | U=4.0 |
| 80–95 | 65–82 | p=0.008** | |
| VPIV/SVL | 66±3.196 | 8±3.607 | U=2.0 |
| 63–70 | 51–63 | p=0.003** |
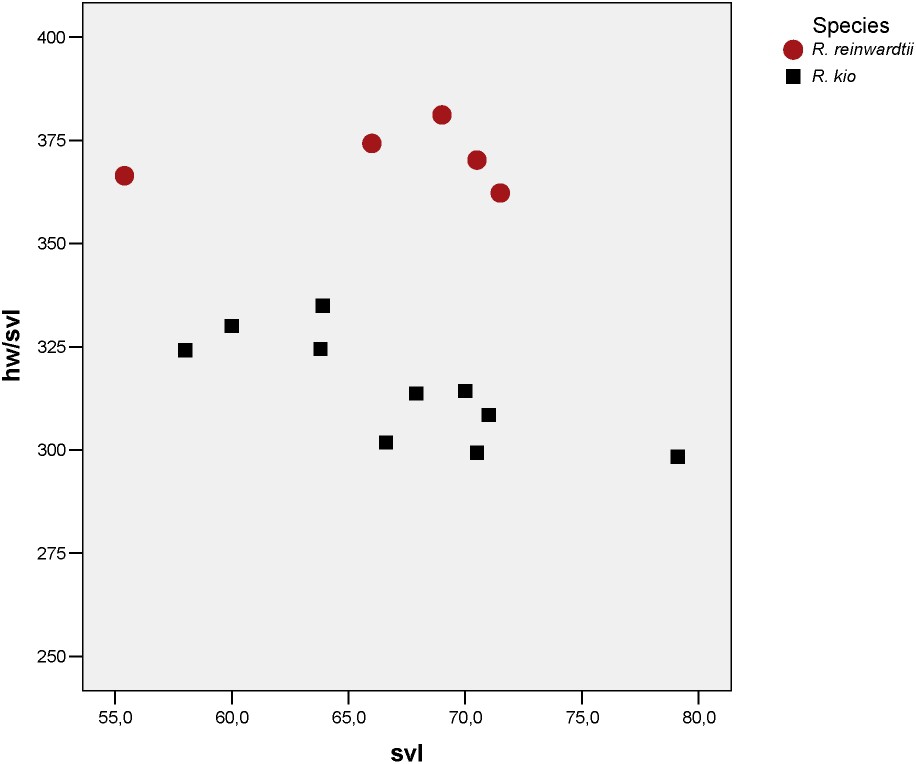
Scatterplot of snout vent length against ratio of head width for Rhacophorus reinwardtii and Rhacophorus kio n. sp.
3.3 Molecular study
The partition homogeneity test did not reject the null hypothesis of congruence of the included gene fragments (1000 replicates; ), thus not contradicting their suitability for combination in phylogenetic analysis. Among the 1323 characters included in the analysis, 799 were constant, 209 variable but parsimony-uninformative, and 305 variable and parsimony-informative.
The maximum-parsimony analysis found one parsimonious tree (1256 steps; consistency index 0.591 retention index 0.452). MODELTEST proposed a (GTR + I + G) model with a alpha shape parameter of 0.454, a proportion of invariable sites of 0.233, and user-defined substitution rates (A–C, 6.211, A–G, 12.502, A–T, 9.835, C–G, 0.000, C–T, 36.561, G–T, 1.000) and base frequencies (, , , ). The ML analysis using the settings proposed by MODELTEST resulted in the tree shown in Fig. 4. The structures of the maximum-parsimony cladograms are similar to these phylograms, the differences relate only to non-constant nodes.
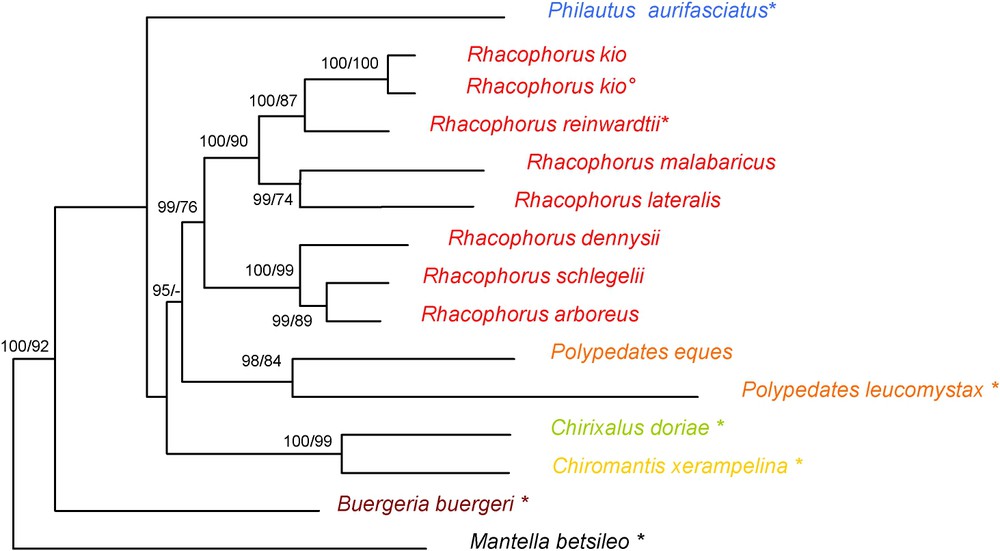
Maximum-likelihood phylogram obtained with all species. It was calculated by PAUP* using a GTR + I + G substitution model selected by MODELTEST, based on 1676 nucleotides of the mitochondrial 12S and 16S rRNA genes. Numbers are Bayesian values and bootstrap values (2000 replicates) of Maximum-Likelihood and Maximum-Parsimony analyses respectively. Values below 50% are not shown. *, type species of the genus; °, holotype of Rhacophorus kio.
4 Description of new species
4.1 Rhacophorus kio n. sp.
Diagnosis. A large-sized Rhacophorus (adult males: 58.0–79.1 mm) with small head and short shanks. Dorsum green colour. Hands and feet fully webbed, web with black spot and orange yellow distal zone. A distinct black marking on flanks. Posterior part of thighs whitish with brown network. Dermal flap on heel pointed.
Onomatophores. Holotype MNHN 2004.0411, adult male, SVL 70.5 mm (Figs. 1 and 5).
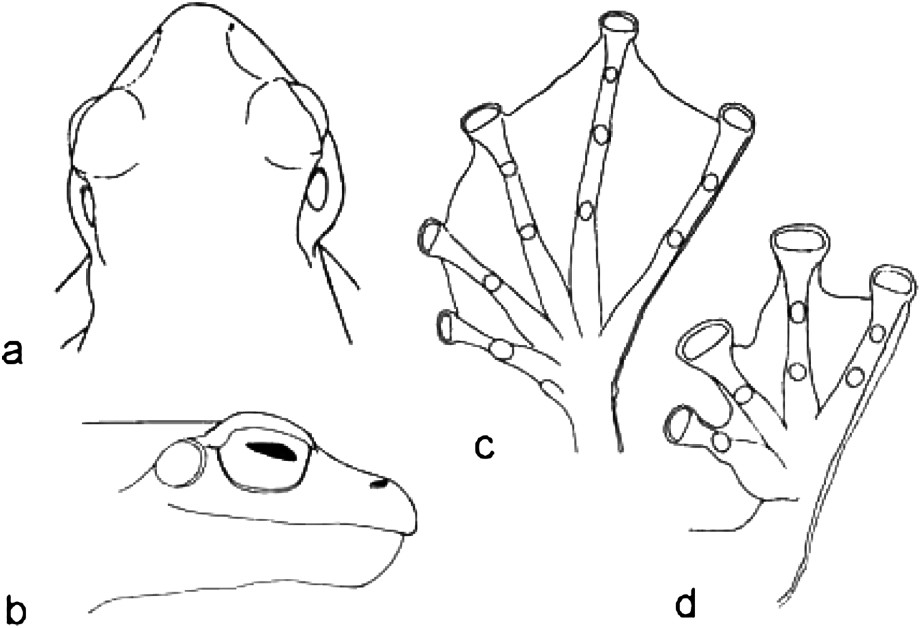
Holotype of Rhacophorus kio n. sp., MNHN 2004.0411, adult male. (a) Dorsal view of head; (b) lateral view of head; (c) ventral view of right foot; (d) ventral view of right hand.
Type locality. Long Nai, Nam Lan Forest Conservation Area, Buon Tai County, Phongsaly Province, Laos.
Description of holotype
(A) Size and general aspect. (1) Specimen of large size (SVL 70.5 mm), body rather slender.
(B) Head. (2) Head moderate, longer (HL 22.5 mm) than wide (HW 21.1 mm; MN 19.2 mm; MFE 14.1 mm; MBE 8.0 mm), flat. (3) Snout pointed, not protruding; its length (SL 11.4 mm) longer than horizontal diameter of eye (EL 7.4 mm). (4) Canthus rostralis rounded, loreal region slightly convex; obtuse in cross section. (5) Interorbital space slightly concave, larger (IUE 7.7 mm) than upper eyelid (UEW 6.0 mm) and internarial distance (IN 5.9 mm); distance between front of eyes (IFE 13.7 mm) about two third of distance between back of eyes (IBE 20.5 mm). (6) Nostrils rounded, without flap of skin; closer to tip of snout (NS 4.9 mm) than to eye (EN 6.7 mm). (7) Pupil oval, horizontal. (8) Tympanum (TYD 4.61 mm), distinct, rounded; tympanum–eye distance (TYE 0.53 mm) one ninth its diameter. (9) Pineal ocellus absent. (10) Vomerine ridge present, bearing rather numerous teeth () small teeth; between choanae, with an angle of 75° relative to body axis, touching choanae, longer than distance between them. (11) Tongue moderate, oval, emarginate; median lingual process absent. Tooth-like projection on mandible absent.
(C) Forelimbs. (12) Arm short, rather thin, fore-arm (FLL 12.5 mm) longer than hand (HAL 23.1 mm), not enlarged. (13) Fingers I short, strong; finger II rather short, rather thin; fingers III and IV long and rather thin (TFL 12.8 mm). (14) Relative length, shortest to longest: I < II < IV < III. (15) Tips of fingers I to IV rounded, enlarged; circum-ventral discs on fingers I to IV, very wide compared to finger width (paI 3.50 mm, waI 1.94 mm; paII 4.99 mm, waII 2.27 mm; paIII 5.25 mm, waIII 2.79 mm; paIV 4.41 mm, waIV 3.11 mm). (16) Fingers with webbing: I 1 1/2–1 II 0–0 III 0–0 IV. (17) Subarticular tubercles present, distinct, rounded, single, all present. (18) Prepollex oval, very prominent; single, bifid, indistinct palmar tubercle; supernumerary tubercles absent.
(D) Hindlimbs. (19) Shanks six times longer (TL 34.2 mm) than wide (TW 5.4 mm), longer than thigh (FL 32.2 mm) and as long as distance from base of internal metatarsal tubercle to tip of toe IV (FOL 34.4 mm). (20) Toes long, thin, toe IV (FTL 18.2 mm) longer than third of distance from base of tarsus to tip of toe IV (TFOL 44.8 mm). (21) Relative length of toes, shortest to longest: I < II < III < V < IV. (22) Tips of toes rounded, enlarged, circum-ventral grooves on toes I to V (ppI 2.59 mm, pwI 1.30 mm; ppII 3.37 mm, pwII 1.62 mm; ppIII 4.02 mm, pwIII 1.62 mm; ppIV 4.15 mm, pwIV 1.81 mm; ppV 3.76 mm, pwV 1.94 mm). (23) Webbing complete: I 0–0 II 0–0 III 0–0 IV 0–0 V (WTF 11.8 mm; WFF 14.0 mm; WI 9.5 mm; WII 11.6 mm; MTTF 23.8 mm; MTFF 27.4 mm; FTFT 9.5 mm; FFTF 9.5 mm). (24) Dermal fringe along toe V from tip of toe along toe, continuing on tarsus to heel, well developed. (25) Subarticular tubercles present, distinct, rounded simple, all present. (26) Inner metatarsal tubercle short, indistinct, its length (IMT 2.76 mm) 3.8 times in length of toe I (ITL 10.5 mm). (27) Tarsal fold absent. (28) Outer metatarsal tubercle, supernumerary tubercles and tarsal tubercle absent.
(E) Skin. (29) Dorsal and lateral parts of head and body: finely shagreened on all parts but lower part of flanks showing treefrog belly skin. (30) Latero-dorsal folds absent; ‘Fejervaryan’ line absent; lateral line system absent; supratympanic fold absent; cephalic ridges absent; co-ossified skin absent. (31) Dorsal parts of limbs: finely shagreened. (32) Ventral parts of head, body and limbs: throat and chest smooth; belly and thigh covered with treefrog belly skin. (33) Macroglands: absent.
(F) Coloration in alcohol. (34) Dorsal and lateral parts of head and body: dorsal parts of head and body and upper part of flank lavender with a few round white spots irregularly set; lower part of flank dark grey network with whitish spots; loreal region, tympanic region and tympanum lavender. (35) Dorsal parts of limbs: lavender; posterior part of thigh beige. (36) Ventral parts of head, body and limbs: throat, margin of throat, chest, belly and thigh and creamy white; webbing ventrally creamy white with greyish indistinct spots on base of web between toes II–V and fingers II–IV; toes and fingers dorsally creamy white, except toe V and finger IV being lavender.
(G) Coloration in life. Dorsal parts of head and body including upper part of flanks grass green with dark green spots, white dots and greenish white markings; lower part of flanks dark brown with yellow spots corresponding to glandular verrucae; a distinct ink black spot in armpit; loreal and tympanic region and tympanum grass green with darker markings; upper lip with a fine white line. Forearm proximally grass green and yellowish, distal part grass green with darker markings. Thigh, shank and feet grass green with dark green bands and markings; posterior part of thigh orange yellow. Throat, vocal sac, chest, belly and lower part of thigh lemon yellow. Webbing orange yellow with a ink black spot at base between toes II–II, III–IV, IV–V and finger II–III, III–IV; toes V and part of IV green. Nuptial spines pinky yellow.
(H) Male secondary sexual characters. (37) Nuptial spines present on prepollex and finger I; indistinct, creamy white forming a unique pad. (38) Vocal sacs present, distinct, unique glandular pouch on throat; slit-like, rather anterior openings. (39) Other male secondary sexual characters: absent.
(I) Sequences. (40) 16S ribosomal RNA gene, partial sequence: 5′–AGC CTG CCC AGT GAT AAA TTC AAC GGC CGC GGT ATC CTA ACC GTG CGA AGG TAG CAT AAT CAC TTG TTC TTT AAA TAG GGA CTC GTA TCA ACG GCA TCA CGA GGG TTA CAC TGT CTC CTC TTT CCA ATC AGT GAA ACT GAT CTT CCC GTG AAG AAG CGG GAA TGA ACT AAT AAG ACG AGA AGA CCC CAT GGA GCT TTA AAC CTC ACA GCA ACT CTA ACA TAT ATT TCC CCA TAA CCC GCA GAG CAA TGC TAG TCG GTT TTA GGT TGG GGT GAC CGC GGA GCA AAA ACT ACC CTC CAC GAC GAA CAG AAC TAA ATC TTT ATC CAA GAG CAA CCA CTC TAA GAA CTA GCA CAC TAA CGT ATC ATG ACC CGA TAA TCG ATC AAC GGA CCA AGT TAC CCT GGG GAT AAC AGC GCA ATC TGC TTC AAG AGC CCA TAT CGA CAA GCA GGC TTA CGA CCT CGA TGT TGG ATC AGG GTA CCC CAG TGG TGC AGC CGN NAC TAA CGG TTC–3′.
Etho-ecology.Rhacophorus kio has been sampled regularly, but the populations were never very large. It has been observed breeding in April in Ban Tup (Bokeo Province, Laos), in July and August in Ben En (Ha Tinh Province, Vietnam) and in July in Long Nai (Phongsaly Province, Laos). Its being rather rare in collection is due to the small size of these breeding populations on the one hand and to the behaviour of this frog on the other. In Long Nai more than ten males could be observed on a tree that covered umbrella-like a large flooded area in a rather well-preserved forest patch. The branches of the tree were exposed to the observer and the treefrogs easily recognizable by their bright yellow vent even though some of them were sitting more than 5 m above the ground. When trying to hit the branches in order to make the frogs fall down, they usually would jump and hide higher in the tree. The method was rather successful in branches of lower height only.
In the ponds in Long Nai where Rhacophorus kio were collected, calling males of Rana bannanica Rao & Yang, 1997 and Chirixalus doriae Boulenger, 1893 could be found on low vegetation near the flooded area. We also could collect a male and female of Rhacophorus bipunctatus Ahl, 1927 in this habitat. In Ban Tup, adult males of Rhacophorus kio were calling on branches of small trees upon a pond where adult specimens of Limnonectes kuhlii (Tschudi, 1838) and tadpoles of Kaloula pulchra Gray, 1831 have been collected. In Sapa, the frogs were perched on the branches of a bush on the border of a small pond where Polypedates leucomystax (Gravenhorst, 1829) and P. mutus Smith, 1940 have been observed breeding; a young Hoplobatrachus chinensis (Osbeck, 1765) was observed in the pond. In the second locality, they were found together with Phrynoglossus magnapustulosus Taylor & Elbel, 1958, Aquixalus odontotasus (Ye & Fei, 1993) and Fejervarya limnocharis (Gravenhorst, 1829).
Distribution. China, Guangdong [25]; Yunnan Province [23,25]. Laos, Bokeo province (hoc loco); Khammouan province [44]; Phongsaly province (hoc loco); Thailand, Doi Chiang Dao, Chiang Mai Province (hoc loco); ‘Me Wang, northern Thailand’ (hoc loco); southern Tak Province [27]; Vietnam, Gia Lai Province [4]; Ha Tinh Province [26]; Lao Cai Province [6]; Quang Binh province [26]; Than Hoa Province (hoc loco).
5 Discussion
Despite the over 5000 species of amphibians recognized, for many people frogs and toads do not have specific names. In science, only a few species of anurans have been widely studied as model organisms, such as Xenopus laevis (Daudin, 1802) or Rana esculenta Linnaeus, 1758. So the presence of the gliding frog, Rhacophorus reinwardtii in general zoology books is worth mentioning. But is the extensive mentioning correlated to intensive taxonomic knowledge?
Rhacophorus reinwardtii is referred to in most systematic and faunistic works on the Oriental region. As its overall morphology is easily recognizable, very few authors studied morphological variation [4,26], but nobody mentioned colour variation as described above. This unawareness is due in part to the concept of the species handed over from the 1930s when the last general revision of the genus Rhacophorus was published, but also to the relative rarity of the large-sized arboreal species in Museum collections. The smaller the samples, the less information is available on intraspecific variation.
In this work, we had access to sufficient material for statistically valid comparisons: by simultaneously studying the type material and topotypical specimens from Leiden, London and Paris herpetological collections, Rhacophorus reinwardtii could be redefined, and by comparing specimens from various localities from the Indochinese Peninsula, a precise concept of the new species could be outlined.
Eventually two rather distinct species emerged, differentiated in several character sets. They can easily be distinguished by colour pattern and morphology. Molecular analysis confirms this differentiation. A reinterpretation of pattern of variation gives a quite clear concept of the two species and will allow every naturalist to recognize them. Their discovery is less a matter of specimens available for study, than a matter of analysis of variation due to a new original view.
The redefinition of these species has implication on biogeography and conservation politics. The distribution area of Rhacophorus kio extends from the most southern place (Gia Lai province, Vietnam, 13°N) to Yunnan and Guangdong (22°N). The most western occurrence is Jonghong (Yunnan, 100°E) (account for West Bengal needs confirmation by voucher specimens). In southern China the distribution area goes east to Longzhou (Guangdong, 107°E). The presence of R. reinwardtii can be inferred from published data as much north as southern Thailand (Ranong [21]; Yala and Narathiwat [27]). The most northern specimen we studied is from Cameron Highland, Malaysia. So the two species must be considered allopatric, as there is no confirmation of sympatry. But Rhacophorus nigropalmatus is sympatric with R. reinwardtii in Malaysia, as confirmed by the specimens studied here. More accurate understanding of the situation will need reanalysis of extant material as it is not always possible to use published data for allocation of names. The zone corresponding to the distribution area of Rhacophorus kio is roughly covering the eastern part of the northern mountain region of Inger [15]. The presence of the more western part (Myanmar, northeast India) must be confirmed by study of specimens.
Amphibians are more strongly threatened and declining than either birds or mammals [45]. The IUCN status of Rhacophorus reinwardtii, as previously understood, is ‘least concern’. The splitting of the distribution area in two rather equal halves will result in a similar appreciation of the conservation status for each of the taxa as the relative distribution ranges are of similar size. We nevertheless must pay attention to the fact that the area of occupancy of these species is much reduced in comparison to the overall surface of south-east Asia (including Sunda Islands) because these species only occur in forest areas. Forest coverage is estimated as low as 28.3% (4275 km2) (but only 5.6% represent frontier forest) [46] and most of the forest left covers slopes of hills and mountains thus not favourable for breeding habitat of these frogs. So the effective forest present in the range of Rhacophorus kio and Rhacophorus reinwardtii is less than 1500 km2 for each species of which about 300 km2 correspond to frontier forest. Deforestation in Asia is still ongoing, so we can expect habitat to diminish. As these species depend on primary forest with well-preserved canopy and on marshes and ponds in or near such habitat for maintenance of populations, they have to be considered as ‘endangered’ according to the IUCN red list criteria. As there are other treefrog species with similar habitat requirements, we can suppose that still more amphibian species should be considered as endangered. This confirms the trends outlined by the first Global Amphibian Assessment [45].
Acknowledgements
We thank the PFCRDP in Phongsaly province, the Provincial and District Officers of Phongsaly province, Frontier Vietnam and IEBR (Hanoi) for supporting field work in Laos and Vietnam. Our acknowledgements are addressed to Thomas Calame and Alexander Teynié from the Alcide-d'Orbigny Society (Clermont-Ferrand, France), Roger Bour, Stéphane Grosjean and Julio Mario Hoyos (MNHN, Paris) who shared fieldwork in Laos and Vietnam. Pim Arntzen (Naturalis, Nationaal Natuurhistorisch Museum, Leiden) Barry Clarke and Colin McCarthy (Natural History Museum, London) are greatly thanked for making specimens available for this study.
Appendix A
Specimens studied
Rhacophorus kio: Laos, Bokeo Province (100°26′E, 20°20′N), Nam Kan Area: MNHN 1997.4092–4093, 1997.4095, adult males; Phongsaly Province, Nam Lan Conservation Area (101°58′E, 21°24′N): MNHN 2004.0411–0412, adult males. Thailand, Mae Wang River [Me Wang, north Siam; several homonymous places in gazetteer]: BMNH 1921.4.1.271, adult female; Vietnam, Thanh Hoa, Ben En National Park (105°45′E, 18°20′N): MNHN 1997.5448–5451, adult males.
Rhacophorus nigropalmatus: Malaysia, Sarawak, Akar River (113°0′E, 2°28′N): BMNH 1947.2.8.89 [ex 1895.7.2.24], holotype, young; Bidi (about 113°30′E, 2°30′N): BMNH 1902.12.12.19, adult female; Cameron Highland, Perak-Pahung border (alt. 3000–4000 ft; 101°27′E, 4°29′N): BMNH 1973.1353, adult male. Indonesia, Boentok, Barito river (114°29′E, 3°32′S): BMNH 1902.1.12.19, adult male.
Rhacophorus reinwardtii: Indonesia, Java: RMNH 1870A, syntype, juvenile, RMNH 1870B, RMNH 3899, RMNH 6517A, RMNH 6517B, syntypes, adult females; BMNH 1891.1.30.12, BMNH 1974.3784, BMNH 1973.1365, MNHN 4605–4606, MNHN 1975.0031, adult females; MNHN 1912.0033–0034, juvenile females; BMNH 1891.1.30.13, BMNH 1974.3783, BMNH 1974.3785, adult males; Jakarta [Batavia] (106°48′E, 6°10′S): BMNH 1845.5.12.18, adult female, BMNH 1845.5.12.19, adult male; Pengalengan (107°34′E, 7°10′S; 4000 ft): BMNH 1896.12.3.29, adult female, BMNH 1896.12.3.30, adult male.
Genbank accession numbers
| Species | 12S I | 12S II | 16S | Geographic origin |
| Rhacophorinae | ||||
| Buergeria buergeri* | AY880458 | AY880478 | AY880444 | Japan |
| Chirixalus doriae* | AF458127 | AF458127 | – | |
| Chiromantis xerampelina* | AY880540 | U22082 | AY880495 | Africa |
| Philautus aurifasciatus* | AY141804 | AY141850 | Java | |
| Polypedates eques | AY880469 | AY880489 | AY920531 | Sri Lanka |
| Polypedates leucomystax* | AY880563 | AY880605 | AY141849 | Vietnam/Java |
| Rhacophorus arboreus | AY880567 | AY880610 | AY880523 | Japan |
| Rhacophorus dennysii | AY880568 | AY880611 | AY880524 | Vietnam |
| Rhacophorus lateralis | AY880569 | AY880612 | AY880525 | South India |
| Rhacophorus malabaricus | AY880570 | AF249029 | AY880526 | South India |
| Rhacophorus kio | AF215188 | AF215359 | Vietnam | |
| Rhacophorus kio° | in progress | in progress | Laos | |
| Rhacophorus reinwardtii* | AY880571 | AY880614 | AY880527 | Malaysia |
| Rhacophorus schlegelii | AY880572 | AY880615 | AY880528 | Japan |
| Outgroup | ||||
| Mantella betsileo* | AY880574 | AY880618 | AY880531 | Madagascar |


