1 Introduction
Proteins involved in metabolic pathways are often distributed non-randomly as multimolecular assemblies that may range from quasi-static, multi-enzyme complexes to transient, dynamic protein associations [1–6]. A functioning-dependent structure (FDS) is such an enzyme complex that forms and maintains itself as a result of its action in accomplishing a task [7]. Moreover, in the case of an extremely simplified model system, we have inferred that such FDSs may display unexpected kinetic properties under steady-state conditions [7]. Before endeavouring to build a complete theory of FDS functioning under realistic conditions, it was necessary to study the behaviour of free (i.e. non-engaged in a FDS) enzymes involved sequentially in a metabolic pathway. This is the aim of the present contribution, in which the calculations have been made according to the classical approach of enzyme kinetics [8].
2 The model of free sequential enzymes
A model of free sequential enzymes of the Michaelis–Menten type is represented in Fig. 1, in the case of a two-enzyme system catalysing the transformation of an initial substrate S1 into a final product S3. This model system is made of two reaction circuits, where the first and second circuits correspond to the activity of the first and second enzyme, E1 and E2, respectively. Clearly, it would be easy to model three-, four-, …, n-enzyme systems by adding a third, fourth, …, nth reaction circuit corresponding to enzymes
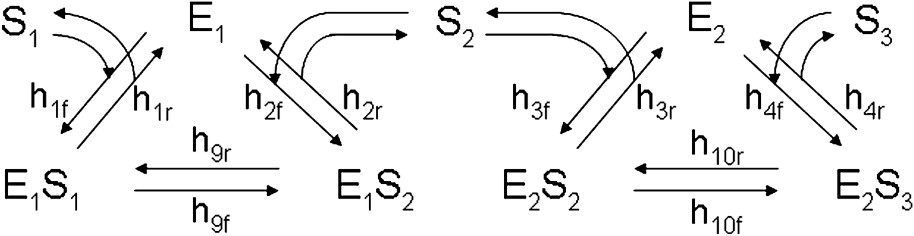
The model of free sequential enzymes in the case of a two-enzyme system. The first enzyme, E1, binds to the initial substrate, S1, to form the enzyme–substrate complex, E1S1. Within this complex, E1 transforms S1 into its product, S2, resulting in the transformation of E1S1 into E1S2, and then E1S2 liberates S2, thus regenerating E1. In similar manner, the second enzyme E2 binds S2; transforms S2 into S3 and finally liberates the final product, S3.
The model of free sequential enzymes in the case of a two-enzyme system. The first enzyme, E1, binds to the initial substrate, S1, to form the enzyme–substrate complex, E1S1. Within this complex, E1 transforms S1 into its product, S2, resulting ... Lire la suite
3 Numerical simulations in the case of a two-enzyme system
The numerical simulations have consisted of studying the dependence of the rate, v, of the overall reaction of transformation of S1 into S3, on the concentration of S1 for various values of the forward and reverse rate constants,
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
In these equations,
To write down the steady-state conditions of functioning of the system, we have assumed that external mechanisms supply S1 and remove S3 as and when they are consumed and produced, respectively, such that S1 is maintained at a constant concentration
| (6) |
Moreover, two relationships have to be taken into account between the rate constants. One is imposed by how the dimensionless quantities have been defined (see Appendix A, Eq. (A.9)),
| (7) |
| (8) |
The numerical simulations of the dependence of v on

Examples of computed curves in the case of a two-enzyme system with
Examples of computed curves in the case of a two-enzyme system with
Increasing
4 Numerical simulations in the case of an n-enzyme system
As an example of an n-enzyme system, we have studied the case of a system made of four free enzymes. We have always found
5 Discussion and conclusion
With a system of two free sequential enzymes of the Michaelis–Menten type in a metabolic pathway, we have always observed in our numerical simulations that the
Acknowledgements
We thank Jean-Pierre Mazat for helpful comments.
Appendix A Definition of dimensionless quantities in the case of a two-enzyme system
Dimensionless quantities have been defined by normalising all concentrations (with [X] = concentration of X) to the sum of the total concentrations of E1 and E2,
| (A.1) |
| (A.2) |
| (A.3) |
| (A.4) |
| (A.5) |
| (A.6) |
| (A.7) |
| (A.8) |
| (A.9) |
Appendix B
The steady-state rate of transformation of S1 into S3 at saturation,
| (B.1) |
| (B.2) |
| (B.3) |
| (B.4) |
| (B.5) |



Vous devez vous connecter pour continuer.
S'authentifier