Version française abrégée
Les petits pélagiques constituent un potentiel halieutique important le long des côtes atlantiques marocaines. Ils sont constitués de sardines, maquereaux, chinchards, anchois et sardinelles. La pêche sardinière est l'une des principales composantes des pêcheries marocaines. Elle constitue 70% de l'ensemble des débarquements. Son activité économique et sociale représente un enjeu considérable, en raison de son implication dans les activités connexes, tant en amont qu'en aval.
L'objectif du présent travail est d'étudier le cycle sexuel de la sardine, de suivre le processus de maturation et de déterminer la saison de reproduction. Il a également pour objectif d'estimer la fécondité partielle, la taille à la première maturité sexuelle et d'étudier le sex ratio.
Parmi les aspects du cycle sexuel étudiés :
- • le sex ratio est le rapport entre le nombre de femelles et le nombre de mâles. La variation du sex ratio en fonction des années et durant la période de ponte maximale a été analysée. Aussi, sa répartition en fonction de la taille a été étudiée ;
- •
la fécondité a été estimée en nombre d'ovocytes hydratés émis par acte de ponte et par femelle mature. La fécondité relative est exprimée en nombre d'ovocytes hydratés par gramme de femelle (sans ovaire). Les valeurs observées de la fécondité sont ajustées à un modèle linéaire (
- •
la taille à la première maturité sexuelle est définie comme étant la longueur totale (
La période de ponte de la sardine a été déterminée en utilisant deux approches :
- • une approche qualitative, basée sur le suivi mensuel du pourcentage des stades de développement macroscopique des gonades. Nous avons utilisé une échelle de maturité sexuelle à cinq stades chez les deux sexes : stade I, immature ; stade II, immature ou au repos ; stade III, pré-ponte ; stade IV, ponte et stade V, post-ponte ;
- •
une approche quantitative, qui consiste à suivre l'évolution mensuelle du rapport gonado-somatique :
Le facteur de condition K reflète les conditions écologiques et physiologiques (maturation et ponte) :
Le sex ratio global ne montre pas de différence significative. Il est en faveur des femelles, qui se regroupent pour se reproduire durant la saison de ponte intense. La répartition du sex ratio en fonction de la taille montre une prédominance des femelles aux petites et aux grandes tailles. Cette dépendance du sex ratio à la taille du poisson a été mentionnée chez la sardine marocaine, chez d'autres populations de sardine méditerranéennes et chez d'autres petits pélagiques, comme la sardinelle du Venezuela et l'anchois de la baie de Cadiz.
La valeur moyenne de la fécondité partielle est de
La taille à la première maturité sexuelle est atteinte respectivement chez les mâles et les femelles à
L'étude combinée des RGS et des stades de maturité sexuelle a montré qu'au niveau de la zone de Laâyoune, la sardine peut se reproduire au long de l'année avec une période de reproduction maximale entre octobre et février. Ce résultat est confirmé par des études d'ichtyoplancton : des œufs de sardine sont collectés durant toute l'année le long de la côte atlantique marocaine ; leur densité est maximale en hiver et devient faible en été. Toutefois, il existe des variations interannuelles du cycle sexuel de la sardine, qui sont dues aux conditions environnementales du milieu, en particulier la température. L'étendue de la saison de reproduction et de ponte maximale dépend de la taille du poisson. En effet, les jeunes reproducteurs (14,5–17 cm) ont une période de reproduction réduite et peuvent se reproduire entre novembre et juin, avec une période de ponte maximale entre novembre et janvier, tandis que les grandes sardines (17,5–25 cm) ont une période de ponte plus étendue, d'octobre à juillet, avec une période d'activité sexuelle maximale d'octobre à février. Ces différences liées à la taille résultent probablement du fait que les jeunes ont encore un taux de croissance élevé et, de ce fait, investissent moins d'énergie dans la reproduction que les poissons âgés.
Le maximum du facteur de condition K correspond au mois (en été) qui précède le démarrage de la reproduction. Cela implique une accumulation de réserves chez la sardine avant la période de ponte, puis un transfert de l'énergie vers la production des gamètes. D'où la relation inverse entre K et RGS. Les variations du facteur de condition observées en atlantique sont en relation avec les indices d'upwelling.
Chez les poissons, le processus de sénilité peut engendrer une diminution de la fécondité ou une diminution de nombre de pontes. Or, notre étude a montré une évolution croissante de la fécondité en fonction de la longueur totale, du poids total (sans ovaire) des femelles. Donc, l'effet de la sénilité n'apparaît pas chez la sardine, qui est une espèce exploitée à longévité courte.
Au Maroc, la reproduction de la sardine a lieu toute l'année, mais, du fait de l'existence du phénomène d'upwelling, qui fait apparaître des variations saisonnières bien marquées, certaines périodes sont beaucoup plus favorables que d'autres. La ponte de la sardine marocaine est donc maximale en hiver, saison d'upwelling minimal et de production zooplanctonique minimale. Elle devient faible en été, saison d'upwelling maximal et de production zooplanctonique maximale.
1 Introduction
Pelagic fish species such as Sardina pilchardus, European pilchardlla spp, Engraulis encrasicolus, Scombrus spp, Trachurus spp constitute important fishery resources throughout the Atlantic coast of Morocco, the first of which remaining the most abundantly caught, reaching 70% of total landings. In these waters, there are three spawning areas for the sardine [1,2]: (1) between Larache and Casablanca, (2) between Tan-Tan and Cap Juby, and (3) between Dakhla and Cap Barbas. Moreover, sardines are considered to belong to three stocks for assessment and management purposes (off northern, central and southern, respectively). Moroccan catches of sardine have been in the order of
The sardine, like most clupeids, is a batch spawner, whose oocytes do not mature simultaneously. Females spawn partially on several occasions within a single season [4].
Previous studies carried out along the Moroccan Atlantic coast on the biology of sardine reproduction concerned either the observations of abundance of eggs and larvae [1,2,5], or temporal distribution of macroscopic stages of gonad development of this species [6]. These studies show that sardine reproduces mainly in winter and secondarily in summer. The hypothesis of a main spawning season in winter and another secondary one in summer was stated.
This present paper consists of an original study of some reproductive characteristics of the Moroccan Atlantic sardine, including maturation process, batch fecundity, size at first maturity and timing of spawning during a three-year period (from January 1999 to December 2001). Interannual fluctuations in these features are discussed. The fecundity was estimated only in January 1998.
2 Material and methods
The studied specimens were collected from commercial landings carried out between January 1999 and December 2001 at the port of Lâayoune, in southern Morocco (Fig. 1). A total of 4644 sardine was sampled, among which 2383 were females and 2261 males. The sampling procedure follows [6]. The sampling rate per week was related to fish availability. Each time, ten specimens were sampled per class of size, measured to the nearest half centimetre. For each specimen, measurements included total length (
Zone of samples selection of Sardina pilchardus.
Sex ratio of males and females is monthly calculated according to the following equation:
Sex ratio
The laying period is determined by two methods: a qualitative method based on monthly changes of the percentage of macroscopic development of gonad stages and a quantitative method based on monthly changes of parameters related to sexual maturity, such as mean gonadosomatic index (GSI) and factor of condition (K).
The gonadosomatic index is defined by [7] as follows:
Size at first sexual maturity (
The logistic model follows [10]:
| (1) |
| (2) |
Some samples of females sardine are collected during the surveys made by the AtlantNiro boat in January 1998, off Laâyoune. One hundred and twenty-three females were used for ovarian histology and 56 others for the estimation of partial fecundity. Mature females at stage IV, ranging in total length between 15.5 and 23 cm and ovaries (without recent post-ovulation follicles) containing hydrated oocytes, were selected for the estimation of the partial fecundity following the gravimetric methods of [4].
Three regions of ovary were cut in the anterior, median and posterior lobe of ovary, respectively [12]. Then, their parts were weighed and preserved in 10% formalin [4]. The oocytes were counted under the binocular. The hydrated oocytes are identified with regard to sizes and transparent aspect. The number of the oocytes was reported to the total mass of the ovary in order to estimate the batch of fecundity (F). The estimated values of fecundity are adjusted to a linear model such as:
3 Results
3.1 Sex ratio
Annual sex ratios were not significantly different from the expected 1:1 ratio, except in 2001 (Table 1). During the period of peak spawning, females outnumber males, but the sex ratio is significantly different only in 2000 (Table 2). Sex ratio by size class (all years) shows a dominance of females in sizes ranging between 9 and 14 cm, except for the class of size 12 cm, where males outnumbered females (Fig. 2). In mean size ranging between 15 and 19 cm, males outnumbered females, except for size 18 cm, where females outnumbered males. In the largest sizes, from 19 cm, the females were the most abundant.
Annual sex ratios of Sardina pilchardus, over the period January 1999–December 2001
| Year | N | Females | Males | Females/Males |
|
P |
| 1999 | 1508 | 787 | 721 | 1.09 | 2.88 | NS |
| 2000 | 1916 | 951 | 965 | 0.98 | 0.10 | NS |
| 2001 | 1220 | 645 | 575 | 1.12 | 4.02 | S |
| 1999–2001 | 4644 | 2383 | 2261 | 1.05 | 3.20 | NS |
|
|
Sex ratio at peak spawning of Sardina pilchardus, over the period January 1999–December 2001
| Year | N | Females | Males | Females/Males |
|
P |
| 1999 | 280 | 146 | 134 | 1.09 | 0.51 | NS |
| 2000 | 429 | 236 | 193 | 1.22 | 4.31 | S |
| 2001 | 270 | 137 | 133 | 1.03 | 0.06 | NS |
|
|

Variation of the sex ratio of Sardina pilchardus with the size, over the period January 1999–December 2001.
3.2 Batch fecundity
The estimated values of fecundity fluctuate between
- • relationship fecundity–total length:
- • relationship fecundity–total weight without ovary:
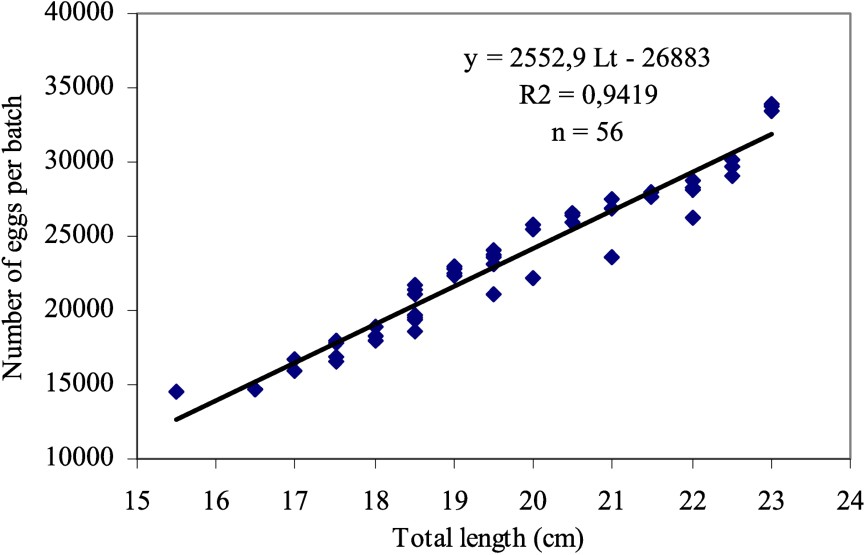
Relation batch fecundity–total length of Sardina pilchardus, January 1998.
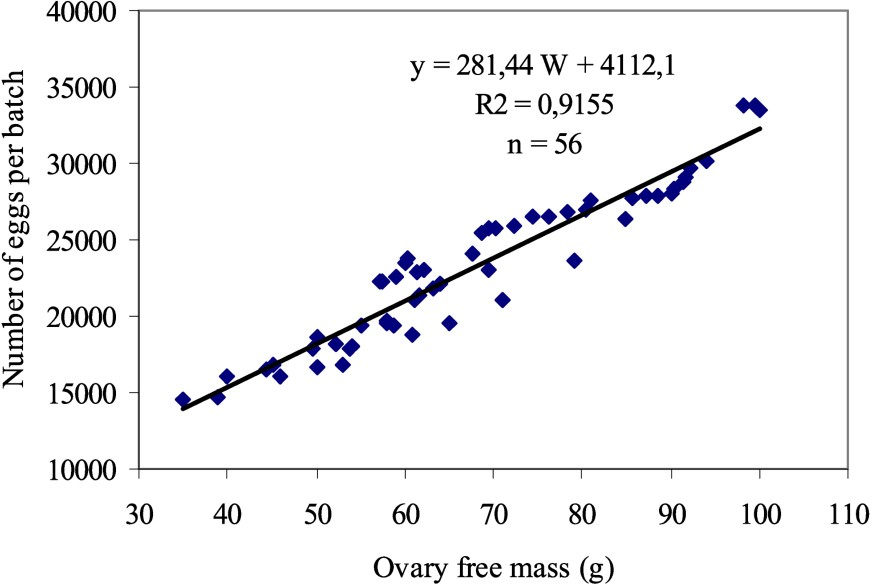
Relation batch fecundity–ovary free weight of Sardina pilchardus, January 1998.
3.3 Size at first maturity
During our study period (from January 1999 to December 2001), length at first maturity (
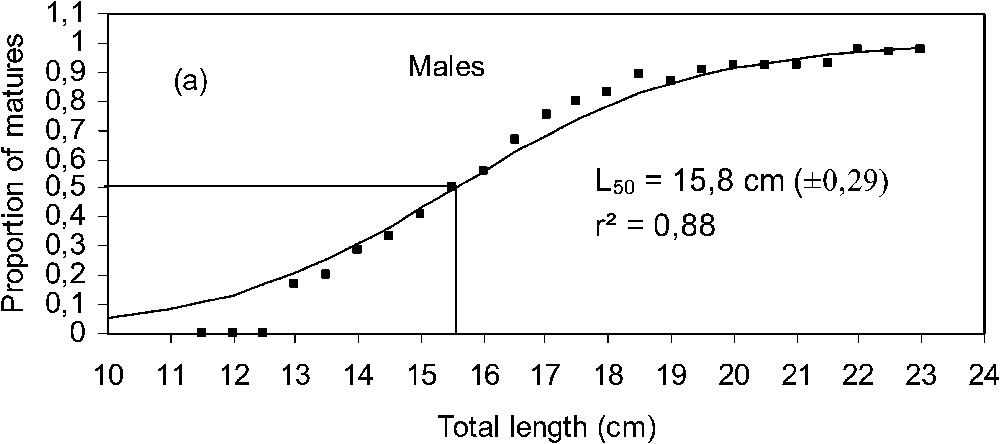

Maturity ogive and length at first maturity (
Annual estimates of total length (cm) at first (
| Years | Males | |||
|
|
|
|
n | |
| 1999 | 15.7 | 21.5 | 0.97 | 483 |
| 2000 | 16 | 20.3 | 0.95 | 864 |
| 2001 | 15.5 | 19.9 | 0.91 | 304 |
| 1999–2001 | 15.8 | 21.3 | 0.88 | 1651 |
| Years | Females | |||
|
|
|
|
n | |
| 1999 | 15.9 | 21 | 0.96 | 648 |
| 2000 | 16.4 | 21.6 | 0.95 | 947 |
| 2001 | 16 | 21.2 | 0.92 | 405 |
| 1999–2001 | 15.8 | 21.2 | 0.87 | 2000 |
ANOVA of total length at first (
| By sex | By year | |||
|
|
F | P | F | P |
| 3.02 | NS (p0.05) | 1.56 | NS (p0.05) |
3.4 Macroscopic stage of maturity
The monthly composition of sardine (male and female) maturity stage from January 1999 to December 2001 (Fig. 6) shows that the spawning season did not start simultaneously for all fish. Pre-spawning (stage III) and post-spawning (stage V) individuals are less represented in samples with variable percentages depending on the year. The percentage of spawning fish becomes important from January to March 1999 and from October to December 1999, from January to June 2000 and from October to December 2000, from January to February 2001, in May 2001 and from October to December 2001 (Fig. 6). By August, most sardines have finished spawning and enter the sexual resting period (stages I and II), except some rare individuals that still could spawn.
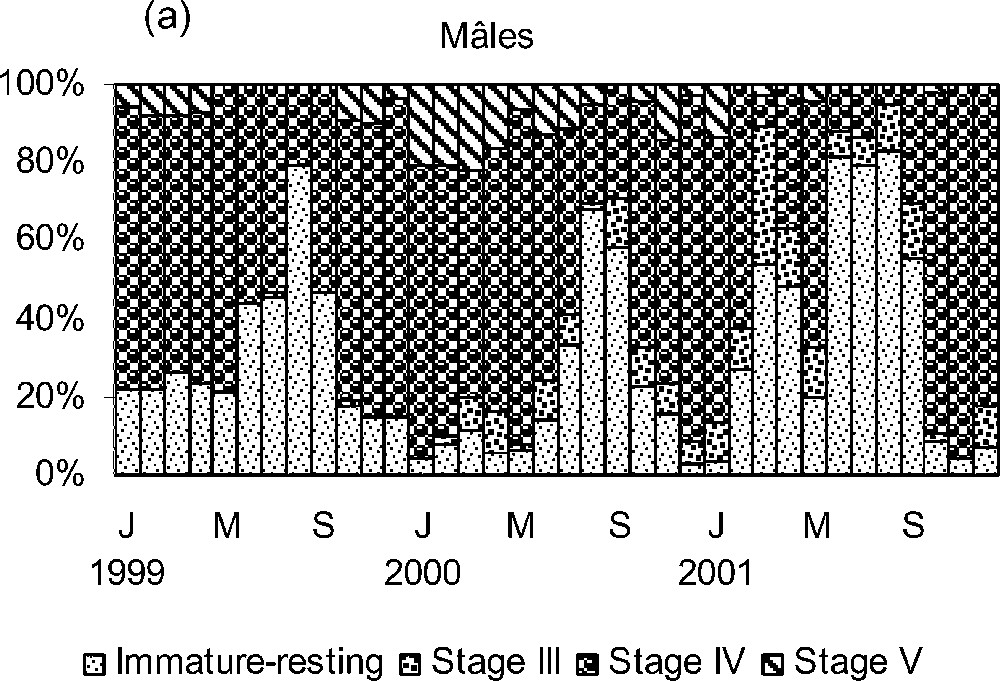

Monthly percentages of macroscopic stages of maturity in (a) males and (b) females of Sardina pilchardus, over the period January 1999–December 2001.
3.5 Gonadosomatic index (GSI) and factor of condition (K)
The evolution of mean GSI of males and females shows similar patterns. The mean values of gonadosomatic index gradually increased from September to December/January and then dropped, reaching the lowest values in summer (Fig. 7). One GSI peak was observed in May 1999, and November 2001, whereas in 2000, the spawning season was more extended with GSI peaks in January and December 2000 (Fig. 7).
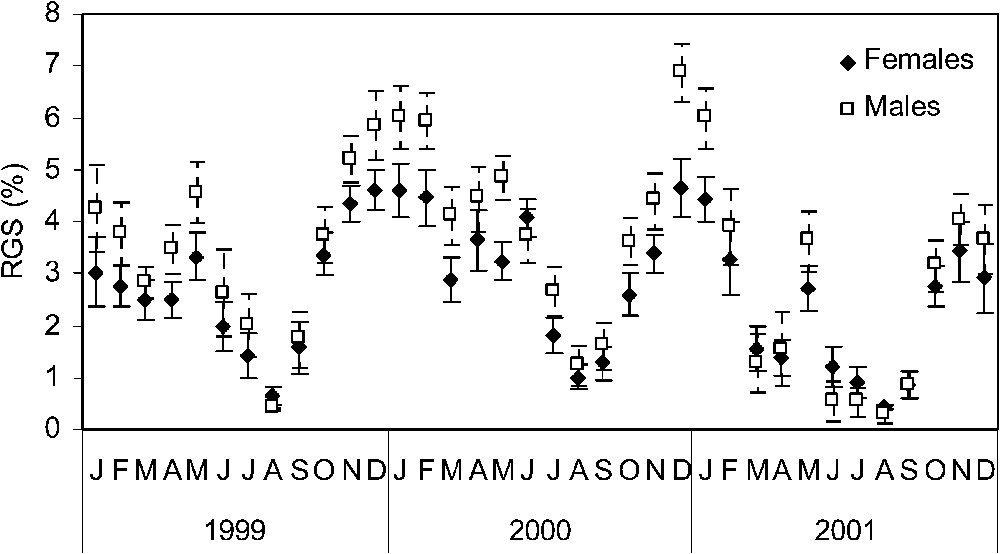
Annual cycle of the gonadosomatic index (mean ± SD) in males and females of Sardina pilchardus, over the period January 1999–December 2001.
The analysis of the seasonal patterns of the percentage of mature sardines (stage IV) (Fig. 6) and mean gonadosomatic index (Fig. 7) indicate the existence of a protracted spawning season that extends from October to July, with a maximal sexual activity between October and February. The sexual resting period takes place between July and August.
The factor of condition K shows similar patterns in both sexes (Fig. 8). From 1999 to 2001, temporal trends of K showed a rather apparent seasonal cycle (Fig. 8), sardines reached a higher condition from June to August and a lower condition from January to April. There was one annual peak in July 1999 and 2000 and two annual peaks in May and August 2001.
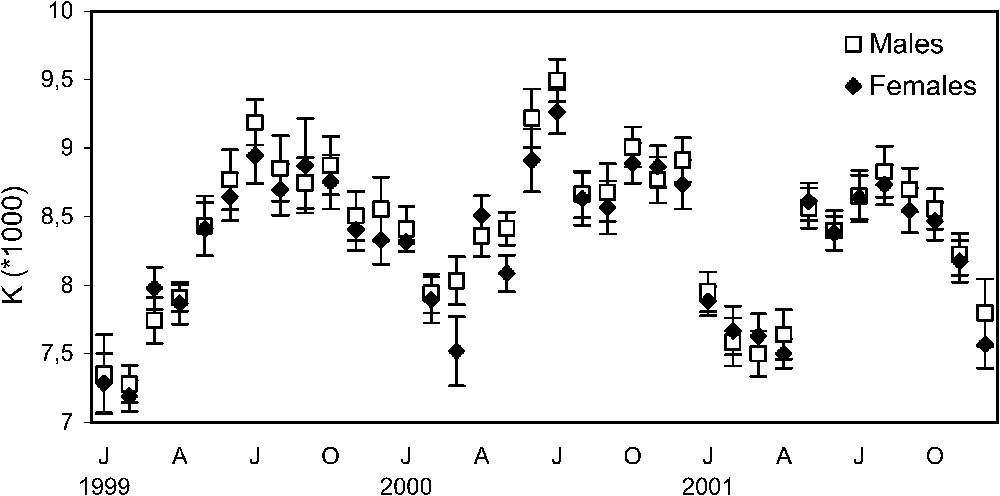
Annual cycle of factor of condition (K) (mean ± SD) in males and females of Sardina pilchardus, over the period January 1999–December 2001.
3.6 Reproductive cycle and factor of condition (K) by size range
Data of both sexes are regrouped because there is no big difference between males and females. GSI and the factor of condition K are calculated by size class.
The duration of the spawning season is probably related to fish size (Table 5). Sardines first spawned at a length of 14.5–17 cm, with a reproductive period from November/January to March/June and GSI peak in November or January (Table 5). Larger fish (17.5–25 cm) reproduce during all the year, except in August 2001, with a GSI peak in May, December or November. Both percentages of spawning sardines (stage IV) (Fig. 6) and GSI peaks indicated that, in the smaller fish, the spawning peak occurs between November and January, and, in the larger ones, between October and February.
Monthly mean (± SD) gonadosomatic index (GSI), by size range of Sardina pilchardus (both sexes), over the period January 1999–December 2001
| Month | 1999 | 2000 | 2001 | |||
| 14.5–17 cm | 17.5–25 cm | 14.5–17 cm | 17.5–25 cm | 14.5–17 cm | 17.5–25 cm | |
| January | 4.01 (2.40) | 1.78 (1.08) | 5.28 (2.19) | 4.98 (2.13) | 4.58 (2.72) | 5.30 (2.10) |
| February | 3.32 (2.01) | 3.12 (2.10) | 4.61 (2.76) | 5.81 (2.17) | 3.48 (2.11) | 3.94 (2.26) |
| March | 2.24 (1.38) | 3.27 (1.94) | 3.57 (2.11) | 3.57 (2.14) | 1.73 (0.68) | 3.46 (2.11) |
| April | 2.66 (1.82) | 3.70 (1.62) | 2.78 (2.12) | 4.32 (2.48) | 0.47 (0.42) | 2.42 (1.98) |
| May | 2.14 (1.83) | 4.58 (1.93) | 2.77 (2.15) | 4.28 (1.83) | 1.08 (0.80) | 3.96 (2.00) |
| June | 1.70 (1.46) | 3.26 (2.08) | 3.82 (2.12) | 3.91 (1.94) | 0.36 (0.21) | 2.19 (1.68) |
| July | 0.85 (0.34) | 2.65 (1.98) | 0.35 (0.15) | 2.73 (2.06) | 0.34 (0.19) | 1.12 (1.01) |
| August | 0.70 (0.56) | 1.40 (1.04) | 0.04 (0.01) | 2.59 (1.78) | 0.34 (0.15) | 0.32 (0.19) |
| September | 1.20 (1.08) | 2.64 (1.46) | 0.83 (0.11) | 3.16 (1.72) | 0.72 (0.43) | 1.06 (0.92) |
| October | 2.12 (1.82) | 4.19 (1.62) | 2.05 (1.72) | 4.38 (1.73) | 2.39 (1.61) | 3.06 (1.54) |
| November | 3.65 (1.34) | 5.06 (1.71) | 1.67 (1.53) | 4.91 (1.63) | 2.90 (1.56) | 3.93 (1.91) |
| December | 3.76 (1.83) | 6.04 (2.14) | 3.58 (2.47) | 6.45 (2.12) | 2.21 (1.93) | 3.69 (1.74) |
In smaller sardines (14.5–17 cm), the maximal annual values of condition appeared in June or July, whereas the minimal values appeared in winter and early spring (Table 6). In larger fish (17.5–25 cm), maximal values are observed from July to October, whereas minimal values are observed in winter and early spring (Table 6). The difference is significantly found between the factor of condition K in both size ranges (ANOVA) (Table 7).
Monthly mean (± SD) factor of condition (K), by range of Sardina pilchardus (both sexes), over the period January 1999–December 2001
| Month | 1999 | 2000 | 2001 | |||
| 14.5–17 cm | 17.5–25 cm | 14.5–17 cm | 17.5–25 cm | 14.5–17 cm | 17.5–25 cm | |
| January | 7.34 (0.83) | 7.10 (0.66) | 8.39 (0.77) | 8.35 (0.63) | 7.68 (0.39) | 7.96 (0.46) |
| February | 7.26 (0.50) | 6.87 (1.59) | 7.81 (0.83) | 8.04 (0.62) | 7.63 (0.68) | 8.11 (0.72) |
| March | 7.86 (0.69) | 8.01 (0.55) | 7.73 (0.86) | 7.89 (0.95) | 7.69 (0.55) | 8.00 (0.57) |
| April | 7.92 (0.56) | 7.89 (0.45) | 8.54 (0.80) | 8.41 (0.55) | 7.80 (0.44) | 7.78 (0.57) |
| May | 8.37 (0.64) | 8.69 (0.68) | 8.19 (0.64) | 8.29 (0.52) | 8.34 (0.46) | 8.97 (0.58) |
| June | 8.35 (0.61) | 8.75 (0.70) | 8.22 (0.60) | 9.04 (0.88) | 8.62 (0.48) | 8.61 (0.44) |
| July | 8.90 (0.70) | 9.24 (0.67) | 9.52 (1.77) | 9.62 (0.59) | 8.60 (0.77) | 9.15 (0.66) |
| August | 8.60 (0.94) | 9.21 (0.84) | 8.11 (0.68) | 9.45 (0.66) | 8.40 (0.94) | 9.23 (0.53) |
| September | 8.34 (0.52) | 9.23 (0.67) | 8.14 (0.60) | 9.30 (1.02) | 8.34 (0.51) | 9.13 (0.47) |
| October | 8.10 (0.84) | 9.06 (0.69) | 8.57 (0.74) | 9.18 (0.65) | 8.35 (0.53) | 8.83 (0.53) |
| November | 8.12 (0.76) | 8.55 (0.69) | 8.43 (0.86) | 9.02 (0.63) | 8.13 (0.96) | 8.47 (0.51) |
| December | 8.19 (0.62) | 8.57 (0.91) | 8.13 (0.84) | 8.96 (0.63) | 7.56 (0.65) | 8.01 (0.58) |
ANOVA of factor of condition K by size range. F: statistical test; p: probability values
| By size range | ||
| Factor of condition K | F | p |
| 9.68 | (p0.01) |
4 Discussion
Sardina pilchardus off Lâayoune is a gonochoric species without sexual dimorphism and presents a sex ratio without significant difference. Similar data are reported by [13] for specimens from the Canary Islands. By contrast, females outnumbered males off the Moroccan northern Atlantic area [6] and in the Alboran Sea [14,15]. Females predominate during the peak spawning season. A similar result was noted by [16] in Sardinella aurita of Senegal. [17] states the existence of reproductive aggregations, which is due to the dominance of females during the period of intensive spawning.
The high proportion of females in the small lengths would be explained by the fact that the ovary is precociously identified. The females outnumbered males in large size classes. Growth of females and males are probably different. This size dependence of the sex ratio was reported in the Moroccan sardine by [6] and other sardine populations by [15,18]. This biologic characteristic was also reported in other small pelagics, like in Sardinella aurita of Senegal [16], in Engraulis encrasicolis of Mauritania [19] and in Sardinella aurita of Venezuela [20].
The fecundity of a population increases according to the size and body weight of fish [21] but, it can vary monthly, e.g., Anchita mitchili of Chesapeake Bay [22] and of S. pilchardus from Portugal [23]. This fecundity can also vary annually and from an area to another, such as in Engraulis mordax and Engraulis ringens [24].
The estimated values of relative fecundity of Sardina pilchardus from Portugal [23,25] are higher than that estimated in the present study (Table 8). Similar values were recorded in European pilchardlla brasilensis from Brazil [26]. High values of relative fecundity estimated for Sprattus sprattus [27] and for European pilchardlla aurita [28].
Relative fecundity of certain species of the family of clupeids
| Species (Clupeidae) | Area | n | Relative fecundity | Authors |
| Sardines | ||||
| Sardina pilchardus | Portugal | 127 | 427 | [25] |
| Sardina pilchardus | Portugal | 422 | [23] | |
| Sardina pilchardus | Lâayoune area | 56 | 346 | Present paper |
| Sardinella | ||||
| Sardinella aurita | Senegal | 400 | [28] | |
| Sardinella brasilensis | Brazil | 23 | 356 | [26] |
| Sprat | ||||
| Sprattus sprattus | Southern North | 41 | 413 | [27] |
The values of the length at first maturity (
At first glance, the inter-annual variability in length at maturity (Table 2) could be attributed to the differential growth of successive annual cohorts when facing different environmental conditions [30].
In the Moroccan Atlantic, the sardine shows a spawning period spread out all year, with an intense sexual activity between October and February. This result is confirmed by the studies of ichtyoplankton [1,2,5]: Sardina pilchardus eggs are collected during all the year along the Moroccan Atlantic coasts, their density is maximum in winter and lower in summer. However, interannual variations in the extent and timing of peak spawning are recorded. These variations seem to be related to changes in physical and biotic conditions [31].
The work carried out authors in the Atlantic Ocean [13,23,32] and the Mediterranean Sea [15,33] over the periods of reproduction of Sardina pilchardus indicates early or late spawning and the existence of a single season of principal spawning that can be spread out over a short or long period of the year according to areas.
The GSI trends by size indicate that the spawning extent and the timing of peak spawning are size dependent (Table 3). Larger fish (17.5–25 cm) reproduce during all the year and are responsible for both the onset and end of the reproductive cycle for the whole population. Their maximum sexual activity occurs between October and February. Conversely, smaller fish (14.5–17 cm) attain first-time maturity later and have a shorter spawning season, their peak spawning occurring between November and January. The interannual differences in the size composition of the stock would result in different combinations of these two size-based reproductive strategies in a given year [17]. Similar size-dependent differences in the maturation process have been observed in other clupeids [15,23].
The mean changes of the factor of condition (K) (periods of fattening and slimming) are contrasted with mean GSI values. This is a common characteristic of Sardina pilchardus from the Mediterranean Sea [15,33,34] and from the Atlantic Ocean [23,29]. The factor of condition K shows that the males develop a strategy similar to that of females in the use of energy contributions during gonadic maturation and spawning.
The maximum mean values of K coincide with the end of the spawning season for the first two years and with the sexual resting phase for the year 2001. These results are in agreement with [15,23,29,33].
In summer, when the trophic conditions are the best, the specimens feed abundantly, while accumulated reserves are in agreement with previous observations [35]. In autumn, phase of gonad maturation, the decrease of mean K could be explained by reserves used for sexual products and moreover the gonads growth depressed the digestive tract of fish [7], and stopped food consumption.
In winter, the zooplankton is less abundant, the fish fed a bit less [36] and the emission of eggs reduces the body mass. This slimming ceases with the resumption of food and the condition improves in spring with a more or less important time shift according to the year. The changes of the factor of condition K observed in the Atlantic are related to upwelling indices [29]. The changes of the upwelling periods influences the spawning periods of clupeid species, such as Sardinella aurita [37].
5 Conclusion
In fish, the process of senility can lead to a reduction in fecundity or a reduction in the number of spawnings. However, our study showed an increasing evolution of fecundity according to the total length, of the total weight without ovary. Thus, the effect of senility does not appear in the European pilchard, which is an exploited species with short longevity [8,38].
The sardine of the Moroccan Atlantic coast has a maximal spawning rate in winter, season of minimum upwelling and minimum zooplankton production. And it has a low spawning rate in summer, season of maximum upwelling and maximum zooplankton production. In fact, small pelagics adopt a spawning strategy that aims at minimising the losses by advection, in order to reproduce outside the season of maximum upwelling [39].


