1 Introduction
Conspecific nest parasitism (CNP) is defined as laying eggs in the nests of conspecific females (or pairs), which then incubate the parasitic eggs and raise the chicks. CNP has been recorded in many bird species [1–4], particularly in precocial species [2,5]. Compared to altricial species, precocial species generally have larger clutches, start incubating only when the last egg is laid, and are probably less limited in the number of offspring they can rear (lower parental care): all these factors are supposed to promote CNP [2,6,7]. High rates of CNP are also found in colonial species, because many nests are found in a small space, presumably increasing the chances of parasitic females to lay eggs in neighbour nests [6,8,9]. Waterbirds from the Laridae and Sternidae are mainly colonial and semi-altricial species: however, in a recent review compiled from the literature of species showing CNP [2], gulls and terns were remarkably little represented, with CNP reported for only six species out of a total of 95. More recently, Duda et al. [10] recorded CNP in the black-headed gull (Larus ridibundus). Moreover, evidence of CNP in these two families was mainly based on field observations of abnormal large clutch sizes and/or abnormal laying sequence (multiple eggs laid in a day or egg laid several days after clutch completion [3,11]).
Evidence of CNP is difficult to get by means of direct observation [1,12], and detection of CNP is most often achieved using indirect methods, i.e. checking the laying sequence for irregularities or looking for variation in the eggs laid by different females (colour, shell pattern, shape, size [13–15]). Recently, molecular techniques (DNA and protein fingerprinting) have been used for detecting CNP [16–18]. However, all these techniques have their benefits, but also their limits [19,20]. In particular, molecular techniques are above all applicable at the chick stage.
In the present study, we examine the occurrence of CNP in a marsh tern species, the whiskered tern (Chlidonias hybrida) that remains a very poorly studied species. The whiskered tern is a cosmopolitan species that is distributed throughout southern and central Europe, but its breeding distribution is scattered, and the species is usually restricted to small colonies established on floating vegetation [21–23]. Given the unfavourable conservation status of the species in Europe [21], the tameness of the species that precludes repeated nest visits, and owing to the fact that a significant proportion of clutches fail before hatching (mainly due to colony desertion when a predator settles close to the colony, or flooding following thunderstorms: authors, personal observations), neither molecular techniques nor daily nest checking (for obtaining the laying sequence) could be used for the whiskered tern. CNP was thus investigated using egg morphology. Nevertheless, a key pre-requisite when using egg morphology data to detect parasitic eggs is the occurrence of a much higher between-clutch variation in egg size than within-clutch variation [24], which ensures that the degree of variation that may exist between one and the other eggs within a clutch [25] will not be greater than the difference between host and parasitic eggs [13,20]. However, the danger of misclassifying an egg as parasitized when it is not exists because the last-laid egg in the clutch frequently looks different from the other eggs of the clutch (it is generally smaller). In the Chlidonias species, conversely to all other tern and gull species studied so far [26–28], females do not lay a smaller last egg compared to the other two in three-egg clutches (black tern C. niger [29], white-winged Tern C. leucoptera [30]).
The present study has four aims. First, we used data from two whiskered tern populations (17 colonies) in France to investigate whether there is a much higher between-clutch variation in egg size than within-clutch variation (a pre-requisite for detecting parasitic eggs using egg morphology data). We also compared the size of the last laid egg in three-egg clutches to that of the other two eggs. Second, we used Eadie's method [31] based on egg morphology (size and weight) to identify parasitized clutches, by calibrating this method to the whiskered tern. Third, we analysed CNP with respect to the clutch size in both studied populations, as the duration of egg laying is a determining factor of CNP rate [32,33]. Fourth, given the possible presence of antiparasite strategies in tern and gull species, i.e. nest guarding and territory defence at the laying and incubation time, we monitored nest attendance of pairs. As it has been suggested that synchrony in laying dates between neighbours in colonially breeding species may be an important ecological factor responsible for CNP [34], we finally report data on synchrony in clutch initiation date in colonies.
2 Methods
2.1 Study sites
We studied whiskered terns in two of the major breeding populations in France, about 200 km apart from each other: the Brenne region (studied in 2001–2004), and the Lake of Grand-Lieu (studied in 2004 only). The Brenne (46°46′N, 01°10′E) covers about 80 000 ha, and consists of a mixture of natural grasslands, forests, and about 2300 artificial fish-ponds that cover approximately 8000 ha [35]. It currently supports 27–40% of the French breeding population of whiskered terns (687–1083 pairs, J. Trotignon, personal communication). In the Brenne, whiskered terns breed on a wide variety of floating and emergent plants [36], though in a restricted number of small- and medium-size ponds each year (on average 15 to 23 ha depending on the years, range = 1–117 ha). Most ponds are owned privately, hence their management depends solely, or mainly on economic needs, i.e. hunting and fish farming [36]. In Brenne, 11 colonies (i.e. 11 ponds that support breeders) were studied: one colony was visited for three years and one colony for two years (see details in Table 1). All colonies were visited only once within the incubation period. Nests were reached by wading, since ponds were shallow enough.
Details on clutches of whiskered tern studied in 17 colonies of two French sites (Brenne and Lake Grand-Lieu) and proportion of clutches assigned as parasitized (%CNP) from egg morphology investigation. One colony was visited for three years (A01, A02 and A04) and one colony for two years (C02 and C04)
| Site | Year | Colony | Conspecific nest parasitism (CNP) | |||||
| Two-egg clutches | Three-egg clutches | > Three-egg clutches | % CNP | |||||
| Non-parasitized | Parasitized | Non-parasitized | Parasitized | Parasitized | ||||
| Brenne | 2001 | A01 | 3 | 0 | 8 | 1 | 0 | 8.3 |
| 2002 | A02 | 5 | 0 | 5 | 1 | 0 | 9.1 | |
| B | 2 | 0 | 9 | 4 | 0 | 26.7 | ||
| C02 | 5 | 0 | 10 | 3 | 0 | 16.7 | ||
| 2003 | D | 7 | 0 | 18 | 3 | 0 | 10.7 | |
| 2004 | A04 | 2 | 1 | 13 | 3 | 0 | 21.1 | |
| C04 | 12 | 0 | 4 | 1 | 1 | 11.1 | ||
| E | 3 | 0 | 6 | 0 | 0 | 0.0 | ||
| F | 5 | 0 | 2 | 0 | 0 | 0.0 | ||
| G | 5 | 0 | 11 | 0 | 3 | 15.8 | ||
| H | 8 | 0 | 5 | 0 | 1 | 7.1 | ||
| I | 2 | 0 | 3 | 1 | 0 | 16.7 | ||
| J | 1 | 0 | 14 | 2 | 2 | 21.1 | ||
| K | 1 | 0 | 2 | 0 | 0 | 0.0 | ||
| Grand-Lieu | 2004 | L | 13 | 0 | 29 | 1 | 0 | 2.3 |
| M | 11 | 0 | 32 | 1 | 0 | 2.3 | ||
| N | 12 | 0 | 16 | 2 | 0 | 6.7 | ||
| O | 4 | 1 | 14 | 1 | 0 | 10.0 | ||
| P | 12 | 0 | 46 | 1 | 4 | 7.9 | ||
| Q | 6 | 0 | 7 | 0 | 0 | 0.0 |
Lake Grand-Lieu is a very large (4000 ha in summer and 6300 ha in winter) shallow, turbid, eutrophic natural freshwater ecosystem in western France (47°05′N, 1°39′W), surrounded by wet grasslands. It is covered with extensive beds of floating plants (770 to 978 ha, depending on the years), comprising mainly waterlily (Nymphaea alba) beds. Currently, Lake Grand-Lieu supports a whiskered tern breeding population of ca. 700 pairs at 10 to 11 colonies (S. Reeber, personal communication) that were well spaced by several hundred metres. Nesting platforms are always built on waterlily beds and consist mainly of waterlily leaves and stems and common clubrush (Scirpus lacustris) stems. Six colonies were visited once a week after clutch initiation in 2004. All nests were accessible by boat.
2.2 Clutch size and egg size
Whiskered terns are known to lay two- and three-egg clutches, the latter being more common [37,38]. The length and width of 1079 eggs from 121 two-egg clutches and 279 three-egg clutches were measured with a Vernier calliper to the nearest 0.1 mm. Egg volume (cm3) was calculated using Coulson's equation [39]. Egg weight was measured using a Pesola® to 0.2 g in Brenne and an electronic balance to 0.1 g in Grand-Lieu. Because the weight of an avian egg declines during incubation (i.e. egg age) as a result of water loss [40,41], we used a linear egg density/age relationship determined on egg data collected at Grand-Lieu for calculating egg age in clutches [38]. This relationship was established from 188 measurements: 70 different eggs of known laying date (32 were measured once, 30 twice and 8 three times totalling 116 egg measurements), plus 72 eggs (measured once) of known hatching date. Based on this relationship, we recorded whether clutches measured in Brenne, and notably two-egg clutches, were complete. We then back-calculated fresh egg weight (i.e. egg weight at laying) using that density/age relationship. The average daily decrease in egg weight was of the egg weight (multiple measurements of eggs, ). Lastly, successive visits performed on Grand-Lieu allowed us to identify the last egg (c-egg) in 11 three-egg clutches, the laying sequence of the two other eggs (a- and b-eggs) being unknown. Finally, based on the density/age relationship, we determined the clutch initiation date (i.e. the laying date of the first egg, see [38]) of each colony of the two study sites as well as the average clutch initiation date (±95% CI, range).
2.3 Identification of parasitized clutches
We used Eadie's method [31] to detect parasitized clutches. The first step in using Eadie's method consists in standardizing all egg measurements (length, width and fresh weight) by colony (z-scores, i.e. mean of 0 and unit variance, see [42]). In the second step, z-scores are used to calculate the Euclidean distance (ED) for the pair of eggs within the two-egg clutches, and the three pairs within the three-egg clutches. Lastly, for each clutch, the largest ED value, MED (maximum Euclidean distance, i.e. the distance between the two most dissimilar eggs), is used to separate parasitized and non-parasitized clutches [31]. Eadie [31] used a cut-off point of 2.5 in MED to identify parasitized clutches for the barrow's goldeneye (Bucephala islandica). Since the within-clutch variance in egg size differs between bird species, a threshold value of MED must be calibrated for the whiskered tern. We used exceptionally large clutches (four-, and five-egg clutches), that were considered to result from parasitized clutches in whiskered tern as they do in similar species [3,11,43,44], for calibration. In the two study areas, 11 such clutches (10 four-egg clutches and one five-egg clutch, providing a total of 70 egg-pairs) were available. In each one of these clutches, the egg (or the two eggs in the single case of a five-egg clutch) showing the highest ED values in all pairs (six pairs within the four-egg clutches and 10 pairs within the five-egg clutch) was considered a parasitic egg. Nevertheless, some caution is needed for using this calculated ED threshold based on supernormal clutches, because the number of egg pairs is limited and we do not have any proof demonstrating that odd eggs in supernormal clutches have been laid by a parasitic female. So, an additional validation of the MED calibration was performed by simulating parasitized clutches (1) by moving one randomly chosen egg (i.e. the parasitic egg) from each three-egg clutch to another randomly chosen three-egg clutch within the data set, or (2) by adding one randomly chosen egg (i.e. the parasitic egg) from each two-egg clutch to another randomly chosen two-egg clutch.
2.4 Behavioural observations
Whiskered terns defend their territories vigorously against conspecifics, including parasitic females [45, unpublished data]. We assumed that parasitic females would lay their egg only when both members of the pair are away from the nest, so we focused on the time during observations where none of the mates were incubating or guarding the nest (“absence time” of both pair members). Since CNP corresponds to multiple eggs laid in a day or eggs laid several days after clutch completion, the study of absence time of both pair members was carried out during the laying and incubation periods. Nest attendance was monitored with a telescope from a distance of 50–100 m on Grand-Lieu in 2004–2005. A total of 135 different nests was monitored before incubation started, i.e. during the laying period (30-min focal each nest; 13 days; 9 May to 6 July) for a total observation time of 4050 min, and additionally 68 other nests were monitored during incubation (12 days; May 26 to July 6) for a total observation time of 2040 min. Nests were randomly selected in 10 colonies to have a representative sampling of bird activity. Observations were carried out between 8–12 a.m. and 3–8 p.m. Sometimes, several absence times were observed during a nest focal. Absence time was classified into five classes: 0 min, 0.01–1.00 min, 1.01–3.00 min, 3.01–5.00 min and 5.01–10.00 min.
2.5 Data analysis
Normality and homoscedasticity were tested using Kolmogorov–Smirnov and Bartlett's tests, respectively. When deviations from normality were detected, non-parametric tests (Kruskal–Wallis) were used. Differences in egg morphology across years in Brenne, and between study sites were tested with multiple analysis of variance (MANOVA and Wilks' λ statistic). Between- and within-clutch variation in egg morphology for each clutch size class was analysed with one-way ANOVA. Fisher's exact test was used to compare %CNP among clutch size classes and sites. The likelihood-ratio chi-square test was used to compare absence times between periods. All statistical analyses were performed using STATISTICA (version 6.0), and 0.05 was taken as the level of significance.
3 Results
3.1 Pattern of variation in egg morphology
There were no differences in any of the egg variables (length, width and fresh weight) between years in Brenne (MANOVA, Wilks' , , ) or between the two study sites (MANOVA, Wilks' , , ). Therefore, all data on egg biometrics were pooled for subsequent analyses. The third egg in three-egg clutches was not smaller than the other two eggs (Table 2). In addition, the between-clutch variation of each egg biometrics was significantly larger than within-clutch variation (see F-statistics in Table 3). From 71.6 to 74.6% and 80.9 to 86.6% in total variation in egg size was accounted for by between-clutch variation in three- and two-egg clutches respectively (Table 3). Therefore, within-clutch variation in egg size was relatively limited compared to between-clutch variation, allowing egg morphology to be used to investigate CNP in the whiskered tern.
Comparison of egg biometrics (length, width and fresh weight) for the c-egg and the other two eggs in whiskered tern three-egg clutches (mean ± SD). Data were collected on Grand-Lieu in 2004 (n=11 clutches). Mean values were calculated for the two first eggs
| Mean ± SD | Kruskal–Wallis test | |||
| Egg biometrics | a- and b-eggs | c-egg | KW | p |
| Length (mm) | 38.37±1.33 | 38.92±1.47 | 50.50 | 0.51 |
| Width (mm) | 27.98±0.52 | 28.07±0.71 | 57.50 | 0.84 |
| Fresh weight (g) | 15.84±0.62 | 16.18±0.95 | 50.00 | 0.49 |
Between and within clutch variation in egg biometrics in two- and three-egg clutches in whiskered terns breeding in Brenne and Lake Grand-Lieu (n=number of clutches)
| Sum-of-squares | n | F | p | ||
| Between clutches | Within clutches | ||||
| Two-egg clutches | |||||
| Length (mm) | 562.85 | 124.18 | 121 | 4.21 | <0.001 |
| Width (mm) | 137.50 | 24.73 | 121 | 5.44 | <0.001 |
| Fresh weight (g) | 362.49 | 56.10 | 121 | 5.51 | <0.001 |
| MANOVA1 | 0.007 | 363 | 4.40 | <0.001 | |
| Three-egg clutches | |||||
| Length (mm) | 1365.92 | 504.30 | 279 | 5.06 | <0.001 |
| Width (mm) | 334.85 | 132.80 | 279 | 6.41 | <0.001 |
| Fresh weight (g) | 913.29 | 310.24 | 279 | 5.91 | <0.001 |
| MANOVA | 0.028 | 847 | 4.60 | <0.001 |
1 A multiple analysis of variance has been performed on the three egg variables together (between and within clutch SS are not presented, Wilks' λ instead).
3.2 Maximum Euclidean distance calibration
Based on values obtained from the supernormal clutches (> three-egg clutches), we found that ED values between non-parasitic egg pairs never exceeded a value of 2.5 (Fig. 1). ED values between non-parasitic egg pairs in the two simulated data sets were similar to those obtained in the observed data set (supernormal clutches), with very few ED values exceeding 2.5 (2.9%, pairs, Fig. 1). Based on these findings, we retained a cut-off point of 3.0 in MED for detecting parasitized clutches because no ED values in non-parasitic egg pairs, whatever the data set used (simulated or observed), exceeded this threshold. Frequencies of parasitized clutches using egg biometry represent therefore minimum estimates for the whiskered tern.
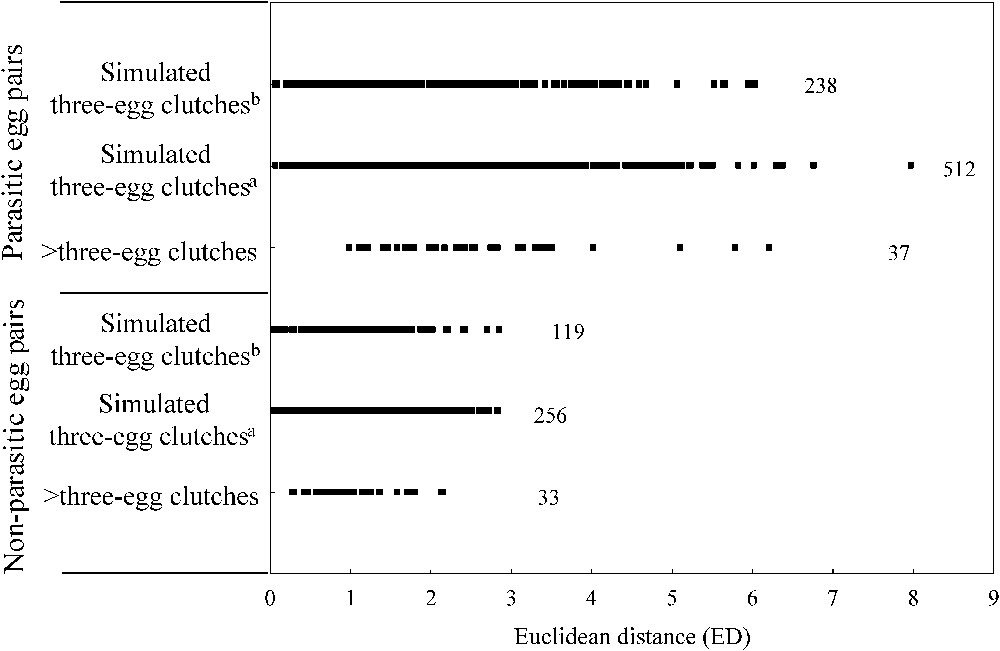
Euclidean distances (ED) calculated for three egg measurements (length, width and fresh weight) between pairs of eggs for whiskered terns in supernormal clutches (> three-egg clutches: 10 four-egg and one five-egg clutches) and simulated parasitized three-egg clutches. Simulated parasitized clutches were obtained (a) by moving one randomly chosen egg among three-egg clutches (n=256), or (b) by adding one randomly chosen egg from each two-egg clutch to another two-egg clutch (n=119). See text for the calculation of ED. Numbers of ED are provided for each clutch type.
3.3 Occurrence of CNP
Out of the 121 two-egg and 279 three-egg clutches, at least two (1.7%) and 25 (9.0%) were parasitized (Table 1). %CNP was significantly higher in three-egg than in two-egg clutches (Fisher's exact test, ). There was no effect of site on %CNP in two-egg clutches (a single case in each site, i.e. 1.6% and 1.7% respectively in Brenne and Grand-Lieu), conversely to three-egg clutches that occurred significantly more often in Brenne (19 clutches, i.e. 14.5%) than at Grand-Lieu (6 clutches, i.e. 4%; Fisher's exact test, ). CNP rates also varied strongly between colonies, in both sites, ranging from 0 to nearly 30% (Table 1). Lastly, no additional egg was ever noted after clutch completion at Grand-Lieu during successive visits to colonies.
3.4 Behavioural observations
Nests were left unattended on average no more than 1 min out of 30 (89.0% of absence time lasted less than 1.00 min whatever the periods, Fig. 2). However, absence time varied between periods (likelihood-ratio chi-square test, , , ). Absence time never exceeded 3 min during the incubation period ( absence times), whereas it was more than 3 min in 4.2% of absence times during the pre-incubation period ( absence times, Fig. 2), and still 2.8% of absence times exceeded 5 min (maximum reached: 10 min). When totalling absence times during each nest focal, 9% ranged between 5 and 10 min during the pre-incubation period ( nests), while it never happened during the incubation period ( nests). Synchrony in laying was very high in all colonies (Fig. 3), with CI of average laying date varying from ±0.90 to 4.08 days according to colonies.
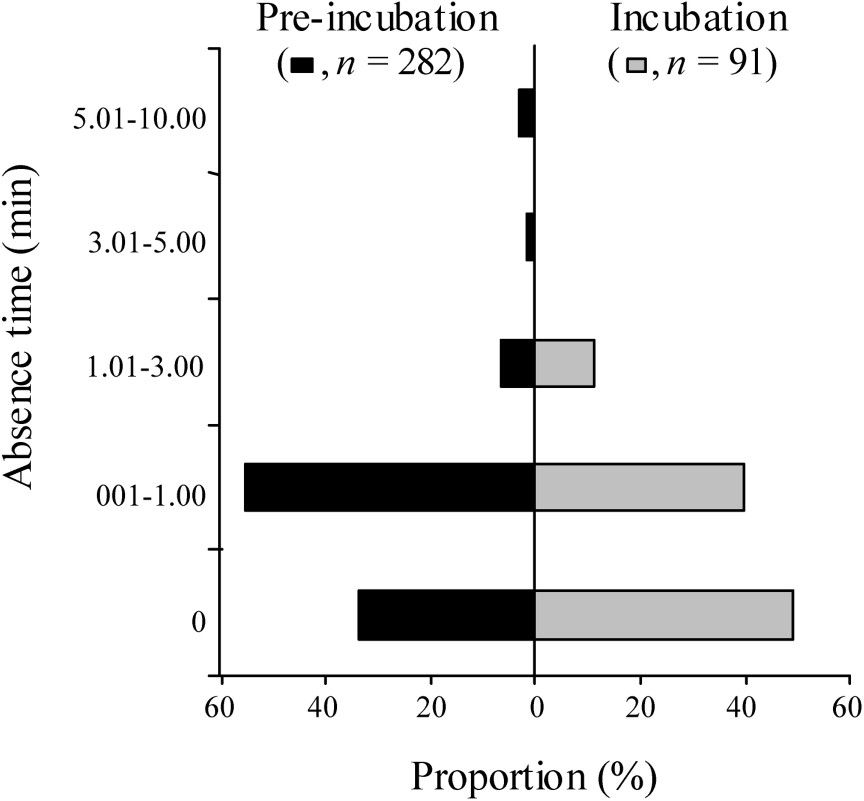
Absence time of both whiskered tern pair members during nest during pre-incubation and incubation periods at Grand-Lieu (n=135 and 68 30-min focal observations respectively). Several absence times can occur during a focal. Numbers of absence time are provided for the two periods.
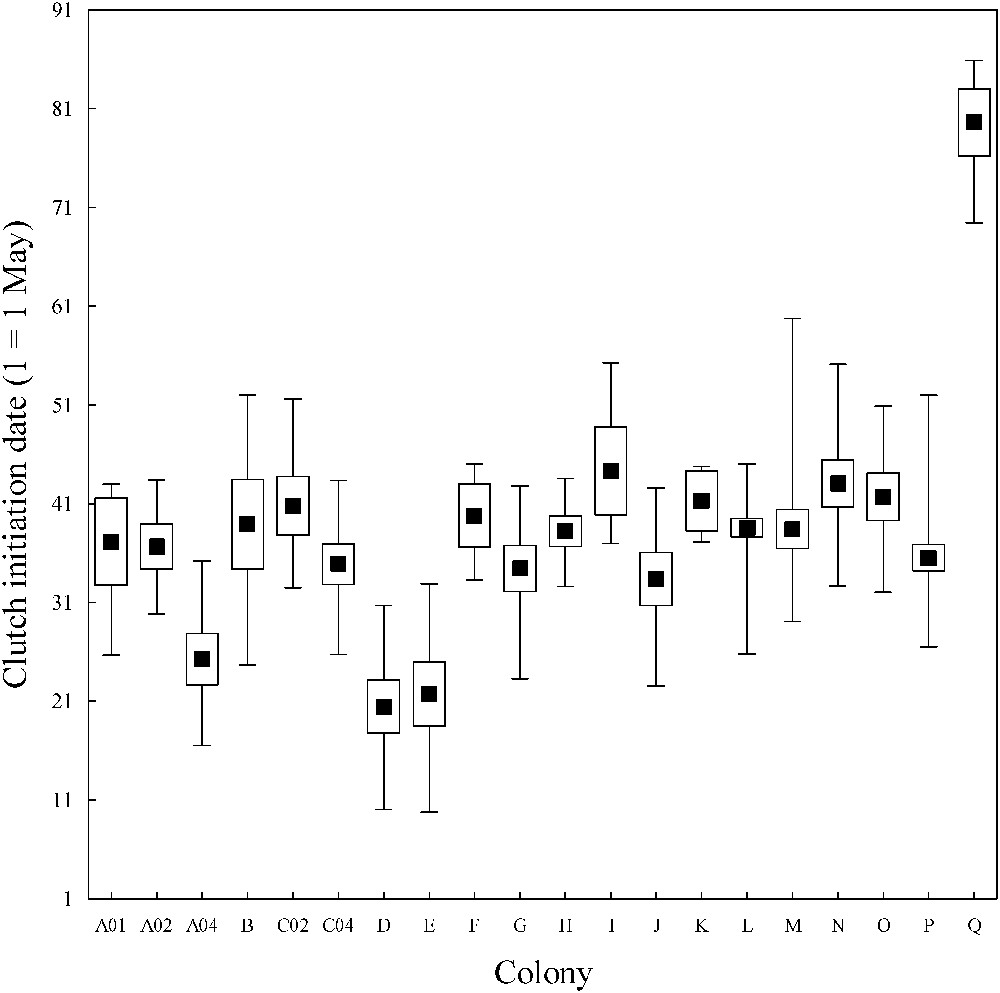
Laying dates within the 17 studied whiskered tern colonies in the two sites. Mean values are shown as black squares, the length of the box represents 95% CI and the length of the bars are the range of values. See Table 1 for colony codes and the text for the calculation of laying dates.
4 Discussion
The use of egg morphology in order to document CNP has been recently debated [13,19,20,24]. For reasons detailed above, we however selected this method in the whiskered tern and verified all pre-requisites when using egg morphology for detecting parasitic clutches. Results showed that ED distributions of parasitized and non-parasitized clutches partially overlapped, an intermediate situation between no overlap as found in some common goldeneye (Bucephala clangula) populations [19] and considerable overlap, for instance, in zebra finch (Taeniopygia guttata), jackdaw (Corvus monedula), and common goldeneye populations [20]. For this reason, our values of CNP in the whiskered tern must be considered as minimum estimates of CNP, and, therefore, relationships between rate of CNP and some colony descriptors such as colony size, nest density, or host characteristics [5,14,31] may be flawed.
To our knowledge, there has been no previous assessment of CNP rate in any tern species. In this study, we found a minimum CNP rate of 9.2% for all clutches combined (). This is a rather high rate in a species with parentally fed young (see reviews in [3,7]). Based on behavioural observations carried out during the study, showing unattendance of nest by any of the pair members during the pre-incubation period, we suggest that this high rate of CNP might result from this facilitating mechanism. Shealer and Zurovchak [11] also explained CNP in roseate tern (Sterna dougallii) by observation of unattended nests. In addition, we found high synchrony in laying dates in whiskered tern colonies, further enhancing opportunities for egg parasitism if parasitic females are able to monitor the nesting cycles of their conspecifics. Such ecological factors have already been correlated to high %CNP in moorhen (Gallinula chloropus) [34]. However, if our behavioural observations may explain why CNP exists in the whiskered tern, it does not explain the observed CNP rate. Conversely to most other tern species [44], whiskered terns build relatively large nest platforms (diameter: 51.1 cm ± 6.4 SD, , unpublished data), therefore implying intensive nest-building duties for breeders [38]. We suggest that some females may optimise their breeding investment in laying eggs in conspecific nests rather than helping males in building large nests. Additionally, whiskered tern nest platforms are notoriously precarious structures, that can be easily destroyed by waves, rising water levels or trampling for instance by coypu (Myocastor coypus). Moreover, at least in some colonies, nest-building material is in short supply and there is much robbing of nest material by neighbouring birds that sometimes results in nest destruction. Parasitic females may therefore be individuals that had lost their nests during the egg-laying period and had to lay somewhere quickly as there is no time to build a new nest. However, several alternative hypotheses exist, e.g. non-nesting females unable to breed for other reasons [9,46], or females trading time and effort between parasitism and parenting [47,48].
We also found contrasted situations with regard to CNP in our two studied populations. The two populations mainly differed in nest density. Nest density is 2.5 times higher in Brenne () than at Grand-Lieu (, unpublished data). Similarly, CNP rate was higher in Brenne than at Grand-Lieu, suggesting a possible link between nest density and CNP rate, as has been found in other bird species ([5,31] for Anatidae; [14] for waders). We also know that larger clutches increase the period during which clutches are sensitive to parasitism [49]. We found that %CNP was higher in three-egg clutches than in two-egg clutches, in support to the latter hypothesis. At present, we do not know if a strategy such as egg recognition (based on colour and spot pattern [50]) and egg ejection is used by hosts in order to counter parasitic females. Given the high rate of CNP found in the whiskered tern, and the higher costs of CNP for hosts (with parental care) to rear a parasitic chick, presumably such a strategy should be developed in this species. This means that variation in egg features among conspecific females should be sufficiently high to recognize accurately parasitic eggs. If it is not the case, clutch-size reduction should be a maladaptive response. Host defences based on conspecific egg recognition are surprisingly rare [50], and further work is needed to determine the degree of variation in colour and spot pattern between conspecific whiskered terns, and the presence of such a defensive tactic.
In conclusion, egg morphology was used to detect CNP in the whiskered tern and provided minimum estimates of CNP. Results showed that CNP is frequent in the whiskered tern (at least 9%). We wish that these preliminary results should encourage new investigations of this reproductive tactic in other tern species.
Acknowledgements
We would like to thank J. Renet, O. Riquet, S. Mortreux and J. Trotignon for their assistance in the field in Brenne. We are also grateful to the ‘Réserve naturelle du lac de Grand-Lieu for giving us access to this site. Part of the study was funded by WWF France, the ‘Région Centre’, the ‘Conseil général de l'Indre’ and the ‘Réserve naturelle de Chérine’ for the Brenne study, and by the University of Rennes and the CNRS for the Grand-Lieu' study. We particularly thank J. Trotignon for having initiated a study on this species in Brenne, for his support and for sharing data. J.K. Christians helped in clarifying some methodological issues. The English style was post-edited by R. Britton.


