1 Introduction
The family Sphyrnidae (Gill, 1862) has two extant genera, Sphyrna (Rafinesque, 1810) and Eusphyra (Gill, 1862). The genus Sphyrna contains eight living nominal species [1,2]: S. (Platysqualus) tiburo (Linnaeus, 1758), S. (Sphyrna) lewini (Griffith & Smith, in Cuvier, Griffith & Smith, 1834), S. (Sphyrna) mokarran (Rüppel, 1837), S. (Mesozygaena) corona, S. couardi, S. (Mesozygaena) media, S. (Sphyrna) zygaena (Linnaeus, 1758), and S. (Mesozygaena) tudes. The genus Eusphyra is monospecific, comprising only E. blochii (Cuvier, 1817). This family of largely pelagic sharks is easily recognized by the lateral expansion of the head, giving its hammerhead aspect, as well as by the placement of the eyes and nasal capsules, in the lateroventral portion of the head. This lateral expansion has resulted in the evolution of a wing-like cephalofoil and is the most expanded of all elasmobranchs.
In Brazil, six nominal species occur especially in the southwestern region [3] (cf., Sphyrna tiburo, S. lewini, S. mokarran, S. media, S. zygaena, and S. tudes).
Sphyrna lewini (Fig. 1A) occurs in the Western Atlantic from New Jersey to Brazil in the Eastern Atlantic from Mediterranean and Senegal to Zaire, in the Indo-West Pacific from South Africa the New Caledonia, in the Central Pacific from Hawaii to Tahiti, in the Eastern Pacific from Southern California to northern Peru [2]; Sphyrna tiburo (Fig. 2A) occurs in the Western Atlantic from North Carolina and exceptionally as far north as Rhode Island, USA, to southern Brazil, also Cuba and the Bahamas, in the Eastern Pacific from Southern California, USA, to Ecuador [2]; Sphyrna tudes (Fig. 3A) occurs in the Western Atlantic from Venezuela to Uruguay [2]; Sphyrna zygaena (Fig. 4A) occurs in the Western Atlantic from Nova Scotia to southern Argentina, in the Eastern North Atlantic from the Mediterranean and British Isles to the Ivory Coast, in the Western Indian Ocean from South Africa to India, in the Western Pacific from Viet Nam to New Zealand, in the Central Pacific in Hawaiian Islands and in the Eastern Pacific from Northern California to Chile [2].
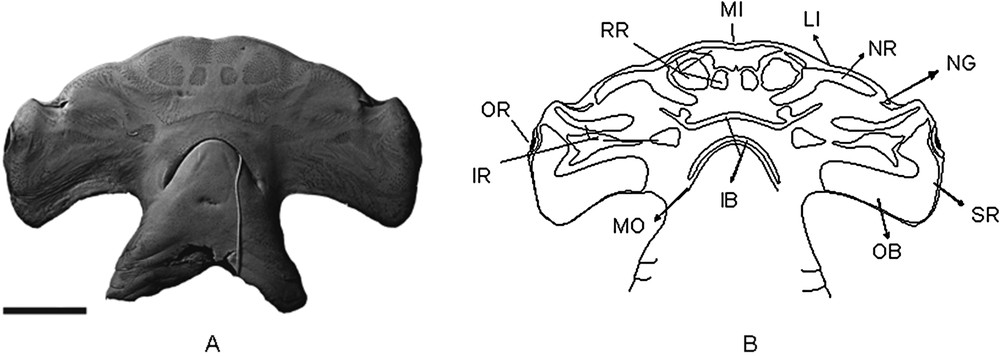
(A) Photograph of the VSC in Sphyrna lewini, showing the clusters of electrosensorial pores (A) and its scheme showing the pore regions (B). AC.UERJ 909, specimen sexually undetermined. Scale bar = 5 cm.
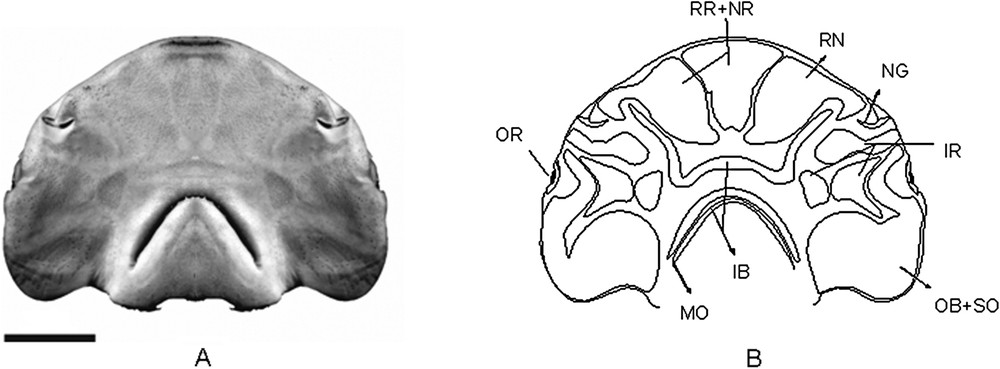
(A) Photograph of the VSC in Sphyrna tiburo, showing the clusters of electrosensorial pores (A) and its scheme showing the pore regions (B). AC.UERJ 864, specimen sexually undetermined, with 10.5 cm of HL. Scale bar = 2 cm.
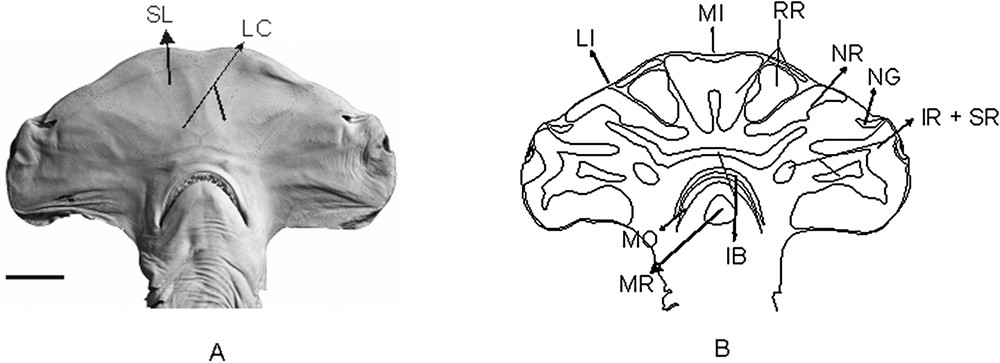
(A) Photograph of the VSC in Sphyrna tudes, showing the clusters of electrosensorial pores (A) and its scheme showing the pore regions (B). MNRJ 537, male with 13 cm of HL and 765 of TL. Scale bar = 2 cm.
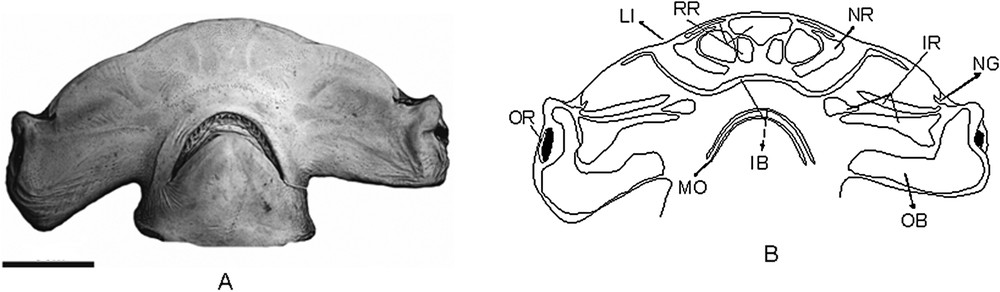
(A) Photograph of the VSC in Sphyrna zygaena, showing the clusters of electrosensorial pores (A) and scheme of the pore regions in S. zygaena (B). AC.UERJ 853, specimen sexually undetermined, with 25.3 of HL. Scale bar = 5 cm.
As the head has special importance in sphyrnid taxonomy and systematics, some authors [4–6] have suggested that more detailed anatomical studies are required, especially on the cranial nerves and brain to detail the neurology and to establish comparisons between the hammerhead species.
During the ontogeny of hammerhead shark species, the head undergoes a lateral expansion principally in the nasal capsules and optic region. It is possible to identify sphyrnid species based only in head morphology [4]. Their heads ranges from evenly rounded and ‘spade-like’ (bonnethead shark S. tiburo) to extremely wide and narrow (winghead shark Eusphyra blochii). Considering that the head shape in sphyrnids changes significantly through ontogeny, identification of species on this character alone can result in misidentification.
In the present study, I discovered a number of cephalofoils in museum collections that were attributed to the wrong species. The identification of species using the distribution patterns of Lorenzini ampullary organs, suggested in this work, together with the head shape, could help to correctly identify the species of those cephalofoils.
Cephalofoil development in Sphyrnidae is still not well understood, with several significantly different hypothesis to explain the evolution of the head in hammerhead sharks. One suggestion is that the cephalofoil evolved as an adaptation for a pelagic life style [7]. Another is that its evolution amplified the visual and olfactive functions of the shark [8,9]. Nevertheless, it must be considered that the pre-branchial lateral expansion in hammerhead sharks allowed expansion of the lateral line and Lorenzini ampullae systems, so increasing the sensorial area in this region [10–12]. The lateral head expansion, with the pectoral fins, also seems to help in the hydrostatic swimming [5,13–15] and the sider spacing of the sensory organs may have increased the precision of chemosensing or electrodetecting prey. It has also been confirmed as a predatory function of the cephalofoil in the great hammerhead shark Sphyrna mokarran over the southern stingray Dasyatis Americana [16] and the spotted eagle ray Aetobatus narinari [17].
The electrosensory system in Chondrychthyes comprises a cephalic network with ampullary organs distributed in the head, capable of detecting very subtle variations in electric fields during hunting and navigation [18]. This sensitiveness to electric fields allows a greater sensitivity during navigation and hunting. The detection of these variations is made by the ampulla-shaped electroreceptor organs, which have ciliated hair cells, filled with a jelly-like substance and synaptic connections in its base [18]. The sensorial function performed by the Lorenzini ampullae is complemented by the mechanosensorial action of the lateral line [18]. Both electrosensory and mechanosensorial ampullae have similar structure and function, formed by hairy cell receptors with an integumentary origin, also filled by a jelly substance and connected by neural synapses to the medullary nucleus to process neural signals [18].
The difference between electroreceptors and mechanoreceptors is in their spatial distribution. The electroreceptors are organized in clusters and also generally restricted to head, while the mechanoreceptors are distributed in the head and trunk [12,19].
Here I describe the pore distribution patterns in the VSC of four different species of Sphyrna. This work represents the first phase of an anatomical revision, with the intention of providing a framework for future phylogenetic studies. Although the literature presents a number of hypothesis of hammerhead shark phylogeny [20,21], no consensus has emerged and the topic remains controversial. The comparative anatomy of the electrosensory system provides an opportunity to test further ideas of hammerhead phylogeny, and also to better understand the life habits between the different species of hammerhead sharks.
2 Material and methods
The present study is based on 4 nominal species of the genus Sphyrna (cf., S. lewini, S. tiburo, S. tudes and S. zygaena). The specimens examined belong to the following institutions: Museu de Zoologia da Universidade de São Paulo (MZUSP), Museu Nacional do Rio de Janeiro (MNRJ) e Universidade do Estado do Rio de Janeiro (UERJ).
Thirty eight specimens of S. lewini were examined comprising 10 males (Head Length (HL) between 12.2 cm and 43.3 cm), 12 females (HL between 11.5 cm and 63.5 cm) and 16 sex not determined (HL between 11.7 cm and 44.3 cm); 21 specimens of S. tiburo, including 12 males (HL between 6.7 cm and 11.0 cm), 8 females (HL between 6.7 cm and 8.2 cm) and 1 sex not determined (HL equals to 10.1 cm); 15 specimens of S. tudes, being 10 males (HL between 4.7 cm and 19.2 cm) and 5 females (HL between 4.7 cm and 5.2 cm); and 8 specimens of S. zygaena, in none of which could the sex be determined (HL between 11.7 cm and 32.3 cm). The non-determination of the sex of the cited specimens is due to the absence of data in the respective collections or to the presence of only the head. The electrosensory pores arrangement in the ventral VSC was studied in all specimens of each species. Photos of the ventral region of the head from a representative specimen of each species were made, as the arrangement of those pores is retained during the development of each species. Whenever the adult of a species was available, it had the preference to be photographed, just because the size of the adult head is larger, so it is easier to visualize the pores.
The patterns found in the specimens were compared with data in the literature. The arrangement of the pore patterns was recorded with their disposition, general diameters and distribution all noted. Following this visual criterion it is easier to study the pore region patterns between all hammerhead species. As observed in all the studied species, the regions are very clear and visually well delimited in both juveniles and adults.
The nomenclature of pore regions in the ventral surface of the cephalofoil (VSC) is based on Chu [22], who described the Lorenzini ampullae and its tubules using the same nomenclature for 73 species of elasmobranches in about 46 genera, including hammerhead sharks (Sphyrna zygaena and S. lewini). Chu considered, in certain species, the appearance and disappearance, development, differentiation, degeneration and reappearance of the Lorenzini ampullae system.
The sphyrnids have, in their VSC, patterns in the position of electrosensory pores. The pattern of the electrosensory pores may have some evolutionary significance, as well as be of use in species identification.
List of abbreviations used in the figures: IB, Inner buccal region; IR, Infraorbital region; LI, Lateral indentation; MI, Medial indentation; MO, Mouth; MR, Mandibular region; NG, Nasal groove; NR, Nasal region; SR, Supraorbital region; OB, Outer buccal region; OR, Orbit; RR, Rostral region.
3 Results
3.1 General description of the pore regions in Sphyrnidae
The distribution of the ampullae of Lorenzini on the ventral surface of the head can be divided in well marked regions, which are maintained during the development of each species. Those regions can disappear or even coalesce depending on species. The nomenclature of the head's regions described here refers to all regions observed in all hammerhead shark species studied, and observations about the morphology and differences between regions are described.
The pores in the VSC in the sphyrnids can be divided into 6 distinct ampullae regions (Fig. 5): rostral, infraorbital, nasal, inner buccal, outer buccal and mandibular.
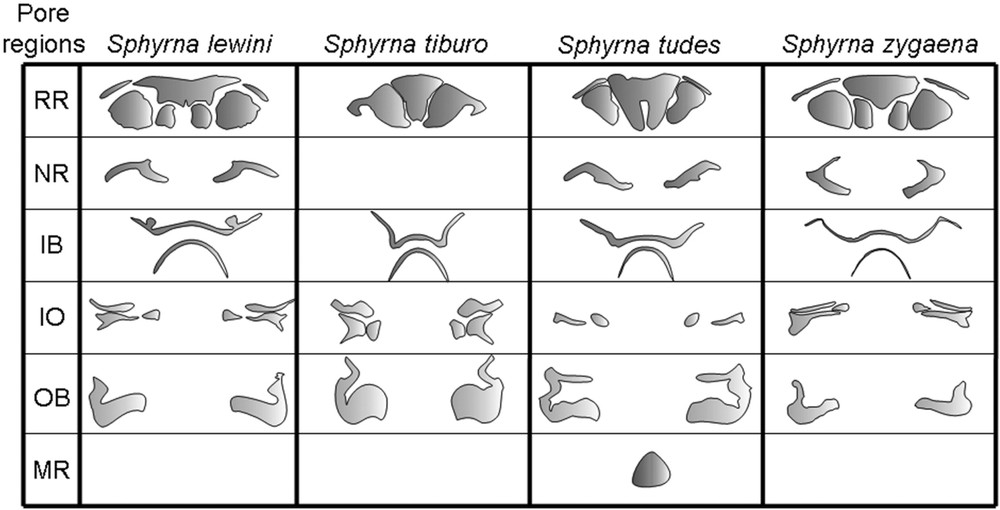
Morphology of the electrosensorial pore regions in the VSC in S. lewini, S. tiburo, S. tudes and S. zygaena. The first column shows, from the top to down, the regions rostral, nasal, inner buccal, infraorbital, outer buccal and mandibular in each species, always when present.
A few synonyms have become apparent when referring to the nomenclature of the pores in the VSC between, respectively, the present work and the literature [7]:
To facilitate the study of pore regions it is recommended to divide them in as few sub-regions as possible. In sphyrnids it is not too complicated because all sub-regions are clearly delineated. Sometimes, as in S. tiburo, these sub-regions there may appear to be two distinct regions. However, when viewed under the stereoscopic microscope, both the large and small pores are mixed in the region, so characterizing a single sub-region. When a region is divided, it must be considered that its principal components, the pores themselves, must form a single distinct region that considers both distribution and diameter.
It is important to note the specific particularities between each region, as this is a relevant characteristic of value for phylogenetic studies of sphyrnids as well as for this family within the Carcharhiniformes, and even for other families in Carcharhiniformes.
3.1.1 The rostral region (RR)
Synonyms: anteromedian region [7] and supraorbital region [22].
This is the most anterior region on the cephalofoil. It is delimited anteriorly by the margin of the rostrum and posteriorly by the prenasal canals, which can be more or less conspicuous depending on the species. It can be divided in one unpaired and central sub-region, which is always present, and 1 to 3 adjacent paired sub-regions according to the species. The unique unpaired sub-region is located well above the subrostral loop (SL), following the rostrum topography and delimited by the rostral cartilages. It has a funnel-like shape, longitudinally larger in its anterior portion. Every paired sub-region is placed adjacent to the unpaired one, and can be found to coalesce with each other or even to other pore regions. This region comprises pores with large and small diameters, but organized in well defined clusters.
3.1.2 The nasal ampullae region (NR)
Synonym: anterolateral region [7].
When present, this region is paired and characterized by a clearly delimited portion, which begins anterior to the nasal groove (NG) and is delimited medially by the margin of the rostral ampullae region.
Its distal portion reaches part of the anterior margin of the rostrum, while its medial portion is adjacent to the margin of the most posterior and distal paired rostral subregion. This proximity to the rostral margin and the rostral sub-region makes the nasal region easily recognizable.
3.1.3 The infraorbital ampullae region (IR)
Synonym: nasal region (anterior part of the infraorbital region) [7].
This region comprises 2 or 3 sub-regions, always paired. All are horizontally aligned being paired in the same horizontal line of the eyes, posteriorly placed in relation to a first, which begins posteriorly to the nasal flaps.
3.1.4 The inner buccal ampullae region (IB)
Synonym: preoral region [7].
This region is characterized by one anterior and one posterior unpaired portion. The anterior one is mostly transversal to the head and located in the median region of the cephalofoil, anteriorly to the mouth (MO). The portion which is not transverse refers to its distal extremities, being turned to the posterior part of the head. On the other hand, the posterior portion rounds the anterior lip of the mouth, usually reaching almost all of its extension.
3.1.5 The outer buccal region (OB)
Synonyms: subocular region (portion reaching the eyes) [7] and posterolateral region (portion reaching the margin of the head) [7].
This region is paired and is the most posterior located of the regions. Its pores fill a greater lateral portion of the head, extending from the eye margin to the posterior margin of the head. Those pores have the largest diameter when compared to the other regions.
3.1.6 The mandibular region (MR)
Synonyms: no synonyms.
This region is unpaired, triangular and located in the median portion of the lower jaw.
3.2 Description of the ampullae regions in the species studied
Sphyrna lewini (Fig. 1B) – As in all observed hammerhead shark species, the rostral region has one medial unpaired sub-region in this species. This sub-region has a “funnel-like” shape, with an almost rectal posterior margin, which has a very subtle medial depression. Its anterior portion has well developed extremities.
It has 3 paired sub-regions: one filled and reaching the anterior margin of the head, between the snout and the lateral indentation (LI); and two rounded and horizontally aligned, posteriorly to the medial unpaired sub-region. From those two rounded sub-regions, the distal one is clearly bigger than the medial. The nasal region is comprised of a paired sub-region with an inverted and well opened “V” shape, thus having three points. The most distal point begins anteriorly to the nasal flaps, reaches the margin of the head until the second point (near to the lateral indentation), continuing until near the distal rostral sub-region.
The inner buccal region is characterized by having paired lobes in its anterior sub-region. Its distal extremities are short.
The infraorbital region has three paired sub-regions, all filled except the most medial one, which is triangular with its distal margin a little pointed.
The outer buccal region is comprised of one unpaired sub-region, large in its extension and comprising pores near at the lateral and posterior margin of the head.
S. tiburo (Fig. 2B) – This is the species with the largest rostral region of all the hammerhead sharks studied, and the only with a single paired sub-region. It contains pores with markedly large diameters anteriorly and some with smaller diameters pores posteriorly.
The nasal region seems to have coalesced with the anterior portion of the inner buccal region.
The infraorbital region has 3 paired sub-regions. The most anterior one is filled. The posterior and medial one is characteristically rounded.
The inner buccal region is characteristic, having its anterior sub-region with the distal extremities expanded a little after the height of the nares.
The outer buccal is well developed, and specially rounded in its medial portion, resembling part of a circumference.
S. tudes (Fig. 3B) – The rostral region has 2 paired sub-regions, one being filled and reaching a small part of the rostral margin and other rounded and adjacent to the nasal regions. The unpaired rostral region is characterized by an elongated depression in its medial portion, and lacks extremities.
The nasal region is comprised of a paired sub-region with three points. The most distal one begins anteriorly to the nasal flaps, reaches the margin of the head until the second point (near to the lateral indentation), then finding the third point of this sub-region reaching the margin of the posterior and distal rostral sub-region. The distance from the second point to the third one is clearly bigger than the first.
The anterior most portion of the infraorbital region seems to have coalesced with part of the outer buccal region. Posteriorly, its distal sub-region is filled, and its medial sub-region is oval.
The inner buccal region has its anterior sub-region with the distal extremities expanded until the height of the nares.
The mandibular region is present, being triangular and located in the medial portion of the lower jaw.
S. zygaena (Fig. 4B) – The rostral region has one medial unpaired sub-region, with a “funnel-like” shape, with a triangular posterior margin and with extremities in the anterior its portion. It has three paired sub-regions: one filled and reaching the anterior margin of the head, between the snout and the lateral indentation (LI); and two horizontally aligned, posteriorly to the medial unpaired sub-region, being the distal rounded and the medial more rectangle shaped. From those two sub-regions, the distal one is transversally larger than the medial.
The nasal region comprises a paired sub-region with “C” shape, with 3 points, very similar to S. zygaena, only differing by its smaller middle point.
The inner buccal region is characteristic, having its anterior sub-region with the distal extremities expanded well after the height of the nares, reaching part of the margin of the head. It extends to the anterior portion of the inner buccal region and seems to be topographically merged with the nasal region.
The infraorbital region has three paired sub-regions, all of them filled except the most medial one, which is triangular with its distal margin a little pointed.
The outer buccal region comprises a single unpaired sub-region, very similar to that seen in S. lewini.
4 Discussion
The identification of hammerhead sharks using the patterns of ampullae of Lorenzini, suggested here, together with the head shape, provides a means to correctly identify the species where only cephalofoils are available. The development of pore region patterns is conservative, meaning those regions are maintained during the ontogeny for each sphyrnids species studied. This pattern maintenance was observed in all available life stages in respectively exemplars among all studied species. In this form, patterns of distribution for the ampullae of Lorenzi can be useful to identify the studied hammerhead shark species in Southwestern Atlantic, with other morphological characters in order to avoid misidentification.
Of course, there are other external characters that must be considered, including size and shape of the fins, position of fourth and fifth gill slits in relation to the insertion of the pectoral fin and the relative lengths of the gill slits and morphology of pelvic claspers [4]. Although, when only the head is available, its shape and the pore patterns of the ventral surface are sufficient to identify the hammerhead shark species from Southwestern Atlantic accurately.
The sphyrnids have, in their VSC, patterns in the position of electrosensory pores. It was observed in the present work that it is important in an evolutionary point of view and also helps to identify all the studied hammerhead sharks from Southwestern Atlantic. It is not clear yet why the sphyrnids have evolved to so different patterns of electrosensorial pores distribution. One hypothesis is that these differences are an evolutionary answer for the adaptation in different life habits between the species.
Comparing the descriptions of the pore regions in the VSC, it is possible to clearly identify the Southwestern Atlantic Sphyrnidae species from the, following a kind of “pore diagnosis” (Fig. 5) based on the distinctive patterns present in each species.
Concerning the pore regions, it is important to note the particularities between the hammerhead shark species studied. There are characters that suggest relationships between the studied sphyrnids, as follows:
Rostral region: S. lewini and S. zygaena have both three paired sub-regions, very similar in its shape, only differing by the shape of the medial paired sub-region. The presence of an anterior filled sub-region reaching the margin of the head approximates also S. tudes from S. lewini and S. zygaena, only differing by the relative size of this sub-region. On the other hand, the unpaired sub-region is distinctive in its shape in each species, clearly allowing them to be separated. S. tudes is the only species that possesses an elongate depression in the unpaired rostral sub-region, and also has the most conspicuous lateral canals.
Nasal region: It is only absent in S. tiburo, in which it seems to be coalescent with the rostral region. The shape of the nasal region is more similar between S. lewini and S. tudes, with an inverted “V” shape, but still can be distinguish in those species. In S. zygaena the shape of the nasal region is more distinct, with a characteristic “C” shape. Besides this region is distinct between the studied species, its presence seems to suggest a close relationship between S. lewini, S. tudes and S. zygaena.
Inner buccal region: The posterior sub-region of the IB is similar in all the studied species. However, the anterior sub-region of the IB can be distinguished in all the studied species by its shape and characteristics. S. lewini is the only one to have paired lobes on it, and S. zygaena is the only one where the extremities of this sub-region pass the height of the nasal flaps, and reach the margin of the head. The shape of the anterior inner buccal sub-region seems suggests a close relationship between.
Infraorbital region: The possession of three infraorbital sub-regions suggests that S. tiburo, S. lewini and S. zygaena could be put together in a related group. However, S. lewini and S. zygaena have both those 3 paired sub-regions with a very similar shape, and distinct from S. tiburo. So, considering that region, S. lewini and S. zygaena would be grouped separated from S. tiburo. S. tudes has only 2 paired infraorbital sub-regions, being separated from all the other species.
Outer buccal region: It is similar in its shape between S. lewini and S. zygaena. S. tiburo and S. tudes but differs by its distinctive outer buccal region shape. S. tudes is the only species presenting a topographical coalescence of this region with the anterior infraorbital sub-region.
Mandibular region: Only S. tudes possesses this region in the studied species.
Gilbert [4] showed superficially the distribution of pores in the VSC in 8 species of hammerheads sharks, among them S. lewini, S. tiburo, S. tudes, and S. zygaena. Although this author concentrated his description on other morphological aspects, he showed in his drawings superficial differences between well distinguished pore regions. However, some problems can be pointed in this work: the small numbers of specimens used for the analysis and the fact that the different ontogenetic stages were not observed. The specimens observed in the present study are not the same as those of previous works [4,23], with the exception of the syntype of S. tudes (a female with 34.6 cm of total length, MNHN 0000-1049), they have the same occurrences.
In the other hand, some authors [24–26] also studied the distribution of electrosensorial pores as a taxonomic tool, as in Rioraja agassizi (Müller & Henle, 1841), Atlantoraja cyclophora (Regan, 1903), A. platana (Günther, 1880) and A. castelnaui (Ribeiro, 1907) [25] and in species of skates of the genus Raja (Linnaeus, 1758) [24,26].
Kajiura [27] described the presence of electrosensorial pores in the inferior lip of S. lewini and S. tiburo, suggesting that both species have another ampullary region reaching the margin of the lower jaw. This region was not confirmed in the present study in S. lewini. Considering that this work [27] did not specify the precedence or even the length or head length of the examined specimens, we should consider that: (a) the pore distribution in the VSC was not well represented; (b) the individuals (juveniles or adults) may represent a distinct subpopulation from outside the Southwestern Atlantic Ocean; (c) eventual taxonomical problems.
The same hypothesis may be valid for S. tiburo, in which the distribution of pore regions of the VSC is different [27] when compared to the observations in the present study.
The pre-programmed number of pores [18] poses the question as to why the cephalofoil keeps growing while the sensorial pores remain of constant number. Its distribution would be an advantage especially to the juveniles, but what about the adults? The proportions between the number of pores and head surface area are not the same for the adults. So, in this work I hypothesize that in adults, with a very advanced stage of life, some pores may be developed to fulfill the empty spaces that the growth of the cephalofoil provides.
In all the species examined here, the distribution of electrosensory pores in the VSC remains constant during the ontogeny, maintaining the same pattern during life. Together with the shape of the head (that changes during ontogeny in different ways according to the species), the pattern of distribution of the pore regions is a very useful taxonomic tool.
It is also important to observe that all those ampullae regions in the studied species are maintained during the shark development.
During the present work, it was observed the holotype specimen of a hammerhead shark species Sphyrna nana [3]. This taxon is considered as a synonym of S. media [2]. The observations of its holotype shows a taxonomical problem, when it is indeed an exemplar of S. media. Although, it was seen two cephalofoils attributed to be from the same species of this holotype. However, the pore distribution patterns are clearly different. In one of those heads, the external morphology is very similar. It was confirmed that this holotype is a S. media, so the species S. nana actually is a synonym of it. Although, considering the clear different pore region patterns in the VSC of the other cephalofoil, this is possible that it is actually from a new hammerhead shark species, or even a subpopulation of S. media.
Nevertheless, the hypothesis that this difference could be a sexual dimorphism can not be discarded, even if it could be the first evidence of it, until now, in hammerhead sharks. The only description, until today, of sexual dimorphism in Sphyrnids is in Sphyrna tiburo, showing differences in the cranial morphology [28]. However, these are very subtle ones.
A study regarding this hypothesis, with more exemplars, can clarify the taxonomical questions regarding to S. media in Southwestern Atlantic. However, the synonymy of these two species seems to be clear.
5 Conclusions
Each species of Sphyrna is characterized by clear distinct pore region pattern in the VSC. These patterns seem to be identical during the ontogenetic development of each hammerhead shark species studied. In this shark, the pore region distribution patterns provide an excellent tool for taxonomical purposes.
The development of pore regions patterns is conservative, with the regions maintained during ontogeny of each sphyrnid species.
These pore regions, their distribution, their sub-regions and the density of sensory organs, provide a useful criterion by which species of hammerhead sharks in the Southwestern Atlantic can be reliably identified. Of course, there are other morphological external characters that must be considered, including size and shape of the fins, position of fourth and fifth gill slits in relation to the insertion of the pectoral fin and the relative lengths of the gill slits and morphology of pelvic claspers [4]. However, when only the head is available, its shape and pore patterns on the ventral surface are sufficient to identify the hammerhead shark species from the southwestern Atlantic with a high degree of precision.
This study shows that S. lewini is very similar to S. zygaena, followed by S. tudes and S. tiburo. This last species appears to be more derived, or at least more distinctive in these sensorial characters.
Acknowledgements
I would like to acknowledge the help of Lucio Machado with the photographs. I also would like to thank the reviewers for their valuable critics and suggestions for improving the manuscript. Thanks to Dr. Paulo Brito for all his help and to Dr. David Martill for its English review and important suggestions. The present work was supported by the CAPES.


