1 Introduction
The impact of grazing on plant communities has long been debated by the scientific community [1–4]. Depending on the frequency and the intensity of the grazing pressure and on the geographical context, this impact may be considered either positive or negative [5,6]. The consequences of grazing on plant communities may be roughly considered as similar for wetlands and dry lands. However, grazing in wetlands presents some specificities related to plant accessibility over time and to the variable inclination of herbivores to go into water [3,7]. In wetlands, a moderate grazing pressure (low density of stocked herbivores) is often used as a management tool for maintaining or restoring the diversity of plant communities [7,8]. The reduction of biomass through removal of vegetation and trampling by grazers is likely to reduce the cover of dominant species [9], and thus to limit competitive exclusion. Moreover, grazing creates niches for plant regeneration [10], allowing the installation of new individuals and species under a lower intensity of competition [11].
Herbivores are likely to alter the species composition and richness of plant communities [3,12] through a differential selection of species [13,14]. They also disperse propagules [15] and thus contribute to increase the species richness of communities and to enhance the connectivity of populations. However, continued heavy grazing pressure reduces the species richness of communities through the consumption of most of the species (weaker selectivity) and increased trampling effects [14,16–18]. Beyond these direct effects, the impact of grazing on species diversity remains dependent on abiotic factors such as climate and soil characteristics [19–21].
Mediterranean temporary pools are endoreic depressions submitted to alternating dry and wet phases and harbouring many rare and threatened species [22,23]. Within their plant communities, annual species (therophytes) constitute a prominent group of plants, able to cope with the large inter-annual variations in water depth and submersion duration [22,24]. The numerous and diverse pools of Morocco are known to inhabit a number of rare and patrimonial species [23,25]. They are intensively used for free ranging grazing [24,26], which is often considered as a major cause of biodiversity losses in Moroccan pools [26,27]. However, the overgrazing of Moroccan pool vegetation, generally considered to have negative effects, contrasts with their present-day species richness. The negative effect of grazing on the plant diversity in pools in Morocco remains largely hypothetical because of the lack of data-based assessment. The scarcity and inaccessibility of ungrazed pools have likely been major obstacles to the assessment of the impact of grazing on pools vegetation. In few hunting reserves created about 30 years ago for commercial game activity, livestock grazing has been forbidden. These areas provide a unique opportunity to attempt a first assessment of the impacts of grazing by comparing the vegetation in grazed and ungrazed temporary pools of Western Morocco.
The main questions posed in this article are:
- • has grazing an impact on the species composition of vegetation of western Moroccan temporary pools?
- • are the species richness and the biomass of plant communities affected by grazing?
2 Material and methods
2.1 Geographical setting
The present study was carried on in the region of Benslimane (western Morocco), located on the Atlantic coast between Rabat and Casablanca. The bioclimate is Mediterranean, semi-arid (annual rainfall: 450 mm) with mild winters (mean daily minimum temperature: 7.5 °C) [28]. Hydromorphic soils are developed on a quartzitic sandstone substrate. The landscape is composed of forests of Quercus suber and of Tetraclinis articulata mixed with cultivated fields and restraint plantations of Pinus spp. and Eucalyptus spp. [28]. This province is characterized by a great abundance of temporary pools, which cover 2% of its total surface area, with a wide range of size, shape, depth, use and location [24,29].
2.2 Regional study
In the Benslimane cork oak forests, 16 temporary pools have been selected (Table 1), 8 of them located within two ungrazed hunting reserves (thereafter named “Ungrazed” pools), and the other 8 in public forests, which are extensively grazed by cattle and sheep (thereafter named “Grazed” pools). In these 2 hunting reserves, grazing has been excluded since 1975. The vegetation of these 16 pools was studied in 2009 at two sampling dates (February and May) and at 2 locations within the pool (the Centre and the Edge), since the species composition varies with hydromorphy [23,25]. At each date and location, phyto-ecological surveys were carried out on homogenous vegetation units (46 m2, corresponding to the minimal area according to Gounot 1969 [30]). These surveys consisted in a vegetation relevé, estimating the cover in 6 classes of each species according to Braun-Blanquet method [31]. The annual or perennial trait was attributed for each species, following the flora of North Africa [32] and Morocco [33]. The water depth was measured in each survey plot at each date.
Characteristics of the 16 studied pools.
| Pool | Latitude N | Longitude W | Altitude (m) | Area (m2) | Type | Dmin-max (cm) | S-Total | S-Annual | S-Perennial |
| F1 | 33°38.088’ | 007°05.830’ | 256 | 10,750 | G | 40–79 | 61 | 43 | 18 |
| F2 | 33°38.381’ | 007°04.957’ | 264 | 2468 | G | 25–70 | 52 | 37 | 15 |
| F3 | 33°38.648’ | 007°05.557’ | 253 | 37,425 | G | 8–55 | 43 | 31 | 12 |
| F4 | 33°38.797’ | 007°05.639’ | 253 | 3933 | G | 0–40 | 44 | 30 | 14 |
| F5a | 33°38.487’ | 007°.05.238’ | 260 | 2964 | G | 14–65 | 46 | 30 | 16 |
| F6 | 33°38.947’ | 007°05.242’ | 256 | 28,633 | G | 0–48 | 61 | 45 | 16 |
| F7a | 33°38.965’ | 007°05.207’ | 257 | 5681 | G | 0–45 | 51 | 39 | 12 |
| F8a | 33°38.547’ | 007°02.876’ | 299 | 1720 | G | 5–50 | 59 | 47 | 12 |
| R1 | 33°41.086’ | 007°04.585’ | 211 | 598 | R | 0–48 | 58 | 43 | 15 |
| R2 | 33°39.290’ | 007°03.770’ | 271 | 3222 | R | 0–30 | 62 | 45 | 17 |
| R3 | 33°38.829’ | 007°03.824’ | 271 | 13,5130 | R | 0–37 | 61 | 47 | 14 |
| R4 | 33°38.824’ | 007°03.248’ | 287 | 5474 | R | 20–50 | 44 | 32 | 12 |
| R5a | 33°41.000’ | 007°05.130’ | 222 | 2405 | R | 40–85 | 49 | 35 | 14 |
| R6a | 33°40.966’ | 007°05.089’ | 223 | 1270 | R | 6–50 | 54 | 39 | 15 |
| R7a | 33°40.944’ | 007°04.961’ | 225 | 6247 | R | 6–50 | 69 | 51 | 18 |
| R8 | 33°38.423’ | 007°08.849’ | 281 | 4334 | R | 25–75 | 45 | 35 | 10 |
a The vegetation of the 6 pools was monitored during 3 years (2007, 2008 and 2009).
The vegetation of each pool was characterized by the number of cumulated species recorded at the 2 dates of sampling for the following categories: the “Total species richness”, measured as the total number of species recorded, “Rare species” calculated as the number of species with patrimonial interest (as defined by [34]), “Pool species” calculated as the number of aquatic and amphibious species that are considered as characteristic of wetland habitat according to North African floras [22,35,36] and to previous works on Moroccan pools [24] and “Opportunistic species” calculated as the number of terrestrial species originating from surrounding forests and agricultural landscapes [32,33] that can establish in the temporary pools during the dry phases. The abundance of Pool species, Opportunistic species and Rare species were calculated for each pool as the cumulative sum of the abundances of the species in each respective group.
2.3 Local study
In order to assess the impact of grazing on the structure of the vegetation, 6 temporary pools (3 Grazed and 3 Ungrazed) were selected among the 16 pools previously studied and were monitored for three years (2007, 2008, 2009). The vegetation was measured 3 times in 2007 (March, May and June) and 4 times in 2008 and in 2009 (January, March, May and June). The vegetation of the 6 pools was studied in quadrats (0.3 × 0.3 m, divided into 9 squares of 0.1 × 0.1 m) which were placed each 2 m along 2 permanent orthogonal transects crossing in the deepest part of the pools. Within each quadrat, the abundance of each species was measured as the number of squares in which it was present (0 to 9). The water depth was measured in each quadrat of vegetation survey.
Within each pool, three plant communities common to all pools were distinguished according to their dominant species, and corresponding to different locations along the topographic gradient (Edge, Intermediate and Centre belts). The communities were respectively dominated by Ranunculus baudotii and Glyceria fluitans in the Centre, by Isoetes velata and Bolboschoenus maritimus in the Intermediate, and by Leontodon saxatilis and Narcissus viridiflorus in the Edge belt. In each of these communities, 5 successive quadrats were selected along the permanent transects. The community structure was then studied within 15 quadrats (0.3 × 0.3 m) for each pool (5 quadrats in each of the 3 topographical levels or 90 quadrats in total over the 6 pools). The number of Annuals, of Perennials and the Total number of species per quadrat (hereafter referred respectively as “Annuals”, “Perennials” and “Total” species richness) were calculated separately for each year cumulating the successive measurements made during the year.
2.4 Biomass measurements
The relationships between species richness and biomass were studied using biomass measurements made in the 6 pools (3 Grazed and 3 Ungrazed) in February 2008, at the expected peak of biomass on Benslimane vernal pools [26]. Biomass was measured in 3 plots (1 × 1 m2) in each community (Centre, Intermediate and Edge; 9 plots in each pool and 54 plots in total) randomly distributed at close vicinity of the permanent transects settled for the study of species composition (see above). The collected biomass from each plot was oven-dried (60 °C) until a constant weight. The species richness (Total and Annuals) of the vegetation nearby biomass plots was calculated using the 3 corresponding (closest) vegetation plots (transects). The species richness was calculated as the cumulated number of species encountered in February 2008 in the 3 quadrats measured.
2.5 Soil analysis
Within each of the 16 pools, two soil samples were collected (10 first cm of soil) respectively at the Edge and the Centre near the vegetation relevés. On these samples, were measured pH, particle size (silt, clay, sand), content in organic matter, total nitrogen, total salinity and total phosphorus. The grain size analysis followed the “Bouyoucos” method [37]. The pH of the soil was measured using a pH-metre in a suspension of fine soil (soil/water in a 1/2.5 proportion). The total salinity of the sediment was measured by electric conductivity (Philipps PR 9801) of an aqueous solution of soil/bi-distilled water (in a 1/5 proportion), previously shaken for 1 hour (150 rpm) [37]. The protocol described by [38] was followed to determine the organic carbon and the total nitrogen (Kjeldahl).
2.6 Data analysis
2.6.1 Study at the regional scale
The relationships between the grazing regime (Grazed/Ungrazed), the environmental variables (water depth, soil characteristics), and the species composition of the vegetation within the pools were studied by multivariate analysis, using the program CANOCO 4.5. The abundance of each species for each pool was calculated as its maximum abundance recorded between the vegetation relevés at the two locations (Centre and Edge) and the 2 dates (February and May). We opted for redundancy analysis (RDA) since previous detrended canonical correspondence analysis (DCCA) indicated a dominance of linear gradients [39]. The statistical power of all tests was assessed by Monte Carlo permutation tests (n = 999). For this analysis, only the species (112) present more than 2 times over the 16 pools have been included in the analysis because of the disproportionate influence of rare species. Forward selection was used, with the aim of determining the local characteristics that explain the main variance in community structure within pools. Only significant explaining variables were retained in the model. Afterwards, variance partitioning was used to compare the amount of variation that was explained either single (alone) or in common with each variable.
The differences in soil characteristics (pH, organic matter, phosphorus, nitrogen, clay, silt, sand, conductivity) and the maximum depth of water between pools within Grazed (8) and Ungrazed pools (8) were tested by variance analysis (ANOVA).
The differences in richness and abundance for Total species, Rare and Pool species between Grazed and Ungrazed pools were tested by ANOVA after check for the distribution of data. The correlations between the total number of species for each pool (Total species richness), the number of Pool species, the number of Opportunistic species (dependent variables), and the maximum water depth and the size of pools (independent variables) were tested using linear regressions. For all statistical analysis (except multivariate analysis), the software JMP4 was used.
2.6.2 Study at the local scale
The influence of grazing regime, of maximum water depth, and of their interaction on the species richness per quadrat (Total, Annuals and Perennials) was analysed using multiple regression separately for 2008 and 2009 (2007 was not considered in the analysis because the hydrology of the pools was similar to 2008). The correlations between the abundance of perennials per quadrat and the number of Annuals and Total species richness, respectively, were analysed using linear regressions separately for Grazed and Ungrazed pools in 2008 and 2009.
The differences of species richness and species abundance per quadrat (Total, Annuals and Perennials) between years (2008 and 2009) according to grazing regime and topographic levels (belts) were tested by variance analysis for repeated measures (MANOVA). The correlations between the total biomass measured in February 2008 and species richness (Total and Annuals) were tested using linear regressions.
3 Results
The 3 years of the study were hydrologically contrasted. The first 2 years, 2007 and 2008, were very dry, with a total annual rainfall of 115 mm (calculated from September to August) in 2007 and 271 mm in 2008, corresponding respectively to 25 and 60% of the annual mean (1961–2006). The 3rd year (2009) was on the opposite humid with 720 mm total rainfall, representing 160% of the annual mean. As a result, the duration of flooding was short for the first 2 years (0–13 weeks) and long for the 3rd year (20–32 weeks).
The maximum water depth, soil pH, conductivity, percentage of clay and sand (Table 2) were not significantly different between Grazed and Ungrazed pools. Ungrazed pools showed significantly higher levels of organic matter, total nitrogen, total phosphorus and silt in the Ungrazed than in Grazed pools (Table 2).
Analysis of variance (ANOVA) comparing the soil characteristics and the maximum water depth in pools exposed to different grazing regime (Grazed/Ungrazed); for each variable, are given the F-value and the probability of different mean values, and for each grazing regime, the mean and standard deviation.
| F | p | Grazed | Ungrazed | |
| pH | 0.12 | NS | 6.05 ± 0.43 | 5.96 ± 0.52 |
| Organic matter (%) | 6.75 | * | 2.82 ± 1.17 | 4.64 ± 1.60 |
| Phosphorus (ppm) | 10.41 | * | 13.58 ± 6.19 | 23.82 ± 5.42 |
| Azote (‰) | 5.96 | * | 1.48 ± 0.59 | 2.32 ± 0.80 |
| Clay (%) | 0.04 | NS | 28.03 ± 12.48 | 28.91 ± 4.60 |
| Silt (%) | 4.90 | * | 24.96 ± 12.73 | 38.07 ± 10.88 |
| Sand (%) | 0.04 | NS | 31.76 ± 16.44 | 33.11 ± 8.19 |
| Conductivity (μs) | 0.25 | NS | 82.56 ± 17.79 | 87.25 ± 19.50 |
| Maximum depth of water (cm) | 0.18 | NS | 56.5 ± 13.53 | 53.13 ± 18.30 |
3.1 Study at the regional scale
Over the 16 pools, a total of 155 species (Appendix A), have been encountered (75% annuals, 25% perennials) including 8 rare species for Morocco: Apium inundatum, Exaculum pusillum, Isoete velata, Myriophyllum alterniflorum, Pilularia minuta, Elatine brochonii, Elatine alsinastrum, and Lythrum thymifolia. Among this total, 23 species were exclusive to Grazed pools, 47 species were exclusive to Ungrazed pools and 85 species were common to both.
The most important environmental characteristics for vegetation (determined by forward selection RDA) are grazing regime, phosphorus content of soil, soil pH, and maximum water depth (Fig. 1). These variables explain 44% of the total variance of the vegetation composition (F = 2.15; p = 0.001). Grazing explains 14.1% of the total variance (F = 2.29; p = 0.001), while the cumulative soil variables (pH and phosphorus) explain 19.6% (F = 1.92; p = 0.003), and maximum water depth explains 9.9% (F = 1.94; p = 0.007). The common variance explained by these variables is weak and not significant (p > 0.05).
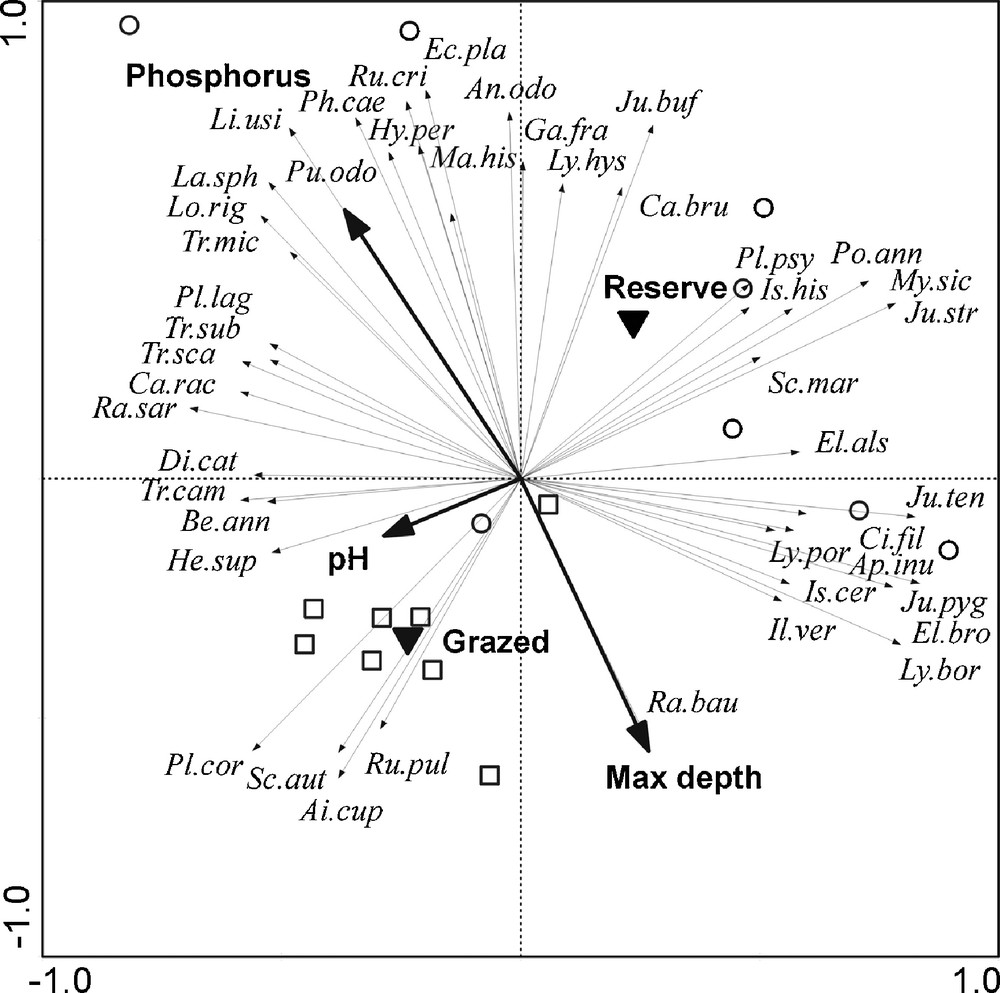
Ordination diagram from principal components analysis (PCA) of the plant species, with environmental factors (grazing regime, soil characteristics and maximum water depth) plotted as supplementary variables (squares: Grazed pools; circles: Ungrazed pools). Only species where more than 28% of the variation is explained by the variables are visualized. For the abbreviation of species, see Appendix A.
A group of exclusive or more abundant species in Grazed pools (Plantago coronopus, Scilla autumnalis, Aira cupaniana, Rumex pulcher…) is identified on the biplot ½ (Fig. 1). This group is opposed to a group of species most abundant or solely present in Ungrazed pools (Elatine alsinastrum, Apium inundatum, Myosotis sicula, Phalaris caerulescens, Rumex crispus, Echium plantagineum…). Most of the species are more abundant in Ungrazed pools (hunting reserves; Fig. 1). The plan ½ (Fig. 1) also separates Pool (aquatic and amphibious) species, which are abundant in deeper pools (Elatine alsinastrum, Apium inundatum, Myosotis sicula …) from Opportunistic species that are more abundant in shallow pools with soils richer in phosphorus (Phalaris caerulescens, Rumex crispus, Echium plantagineum, Linum usitatissimum).
The Total number of species per pool, the number of Pool species and the number of Rare species do not vary significantly between the two grazing regimes (p > 0.05). However, the total abundance of Pool species is significantly lower in Grazed than in Ungrazed pools (F = 11.32; p < 0.01). The abundance of Rare and Opportunistic species is not significantly different between the two pool types (p > 0.05). The Total number of species within each pool, the number of Pool species and the number of Rare species are not correlated with maximum water depth or with the size of pools (p > 0.05).
3.2 Study at the locale scale
In 2008, the number of species per quadrat (respectively Total, Annuals and Perennials) decreased significantly with the maximum water depth (Table 3) but did not show significant differences between the grazing regimes. In 2009, the water depth had a similar significant effect on the Total species richness and on the number of Annual species, but was not correlated to the number of Perennial species per quadrat. The number of species per quadrat was significantly higher in Ungrazed than in Grazed pools (Table 3).
Multiple regression analyses of the effect of grazing regime (TY: Grazed/Ungrazed), of maximum water depth per quadrat (MD) and of their interaction (TY*MD) on the total species richness, the Annual species richness and the Perennial species richness in 2008 and 2009.
| Richness | TY | MD | TY*MD | |||
| F | P | F | P | F | P | |
| 2008 | ||||||
| Total | 0.65 | NS | 19.51 | *** | 0.36 | NS |
| Annuals | 1.50 | NS | 15.44 | ** | 0.08 | NS |
| Perennials | 3.35 | NS | 17.49 | *** | 1.59 | NS |
| 2009 | ||||||
| Total | 9.75 | ** | 16.94 | *** | 3.19 | NS |
| Annuals | 5.58 | * | 16.47 | *** | 3.36 | NS |
| Perennials | 6.10 | * | 2.54 | NS | 0.32 | NS |
The Total number of species and the number of Annual species per quadrat (MANOVA, Table 4) differed significantly between Grazed and Ungrazed pools, along the hydromorphic gradient and between years. The number of Perennial species per quadrat was significantly different between belts (topographic gradient) and between years, with a significant effect of the interaction between belts and pool types. Similarly, the vegetation abundance in the quadrats (Total and Annuals) was significantly different between the pool types, between belts and between years (Table 4). The abundance of Perennial species per quadrat was significantly different between belts and between pool types, with a significant interaction between pool types and years. The abundance of Perennial species was significantly higher the 2nd year in the Central and Intermediate belts in Ungrazed pools. Conversely, it was significantly lower in all belts in Grazed pools. The pool type had a significant effect on the abundance of all groups of species, with a significant effect of belt and time (except for Perennial species) (Table 4).
Analyses of variance for repeated measurements (MANOVA) of the effects of grazing regime (TY: Grazed/Ungrazed), belt, time, and their interactions on species richness and species abundance.
| Type (TY) | Belt (B) | Time (T) | TY*B | TY*T | B*T | |||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Richness | ||||||||||||
| Total | 7.99 | ** | 37.81 | *** | 154.96 | *** | 3.71 | * | 4.22 | * | 14.11 | *** |
| Annuals | 9.51 | ** | 37.76 | *** | 137.29 | *** | 2.54 | NS | 10.75 | ** | 10.75 | *** |
| Perennials | 0.92 | NS | 11.53 | *** | 47.78 | *** | 3.17 | * | 3.55 | NS | 7.66 | ** |
| Abundance | ||||||||||||
| Total | 26.33 | *** | 18.34 | *** | 73.36 | *** | 1.05 | NS | 0.02 | NS | 11.85 | *** |
| Annuals | 22.41 | *** | 17.88 | *** | 103.37 | *** | 1.50 | NS | 1.93 | NS | 9.91 | *** |
| Perennials | 9.07 | ** | 4.82 | * | 0.48 | NS | 2.48 | NS | 9.79 | ** | 4.61 | * |
In 2008, the richness in Annuals and the Total species richness per quadrat were positively correlated with the abundance of Perennial species per quadrat in Grazed pools (r2 = 0.28; p < 0.05; n = 45 and r2 = 0.44; p < 0.05; n = 45, respectively for the Annuals and the Total species richness) and in Ungrazed pools (r2 = 0.23; p < 0.05; n = 45 and r2 = 0.35; p < 0.05; n = 45 respectively for the number of Annual species and Total species richness). In 2009, the Annual species richness per quadrat was not significantly correlated to the abundance of Perennial species in both Grazed and Ungrazed pools (p > 0.05). The Total richness was weakly correlated to the abundance of Perennial species in the Grazed pools (r2 = 0.14; p < 0.05; n = 45) and in the Ungrazed pools (r2 = 0.22; p < 0.05; n = 45).
3.3 Biomass
The biomass in February 2008 was significantly higher in Ungrazed (123.15 ± 25.81 g/m2) than in Grazed pools (42.26 ± 23.28 g/m2). The Total species richness and the number of Annual species per quadrat were weakly correlated to the total biomass of vegetation (Fig. 2).
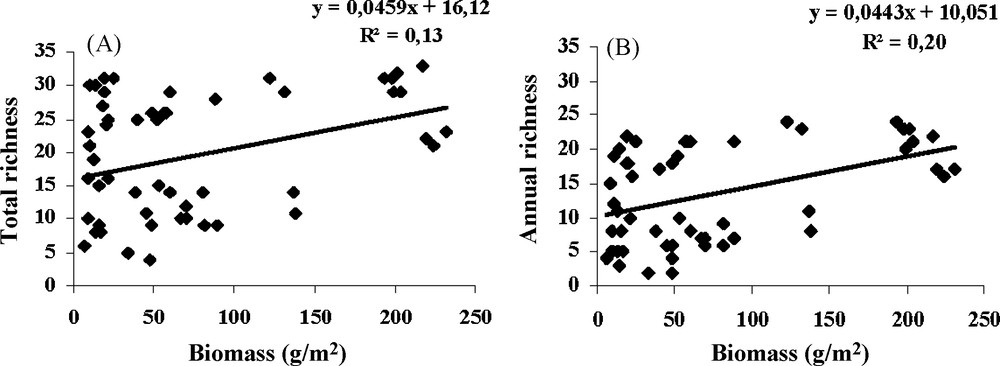
Correlation between the total biomass and (A) the Total species richness, (B) the Annual species richness in the 6 pools combined (Grazed and Ungrazed).
4 Discussion
This study is, to our knowledge, the first attempt to assess quantitatively the impact of grazing on the vegetation of Mediterranean temporary pools. The highly aggregative distribution of ungrazed pools constrained the sampling design of this study. This was imposed by the rarity of such uncommon land use (hunting reserves) in North Africa, while in contrast all natural landscapes were overgrazed. However, the proximity of the Grazed and Ungrazed pools in our study, the similarity of their physical environments and the duration of the monitoring over 3 years support the allocation to grazing effects of the differences measured between Grazed and Ungrazed pools. Indeed, both pool types show many similarities, including the most important variables for pool vegetation [25]: land use in the catchment (forests), nature of the underlying rock (sandstone-quartz), maximum water depth and soil characteristics (pH, granulometry and conductivity). The only differing characteristics of soil were the content in phosphorus, nitrogen, and organic matter, which were significantly higher in Ungrazed pools than in Grazed pools (Table 2). These differences may be attributed at least partly to the absence of grazing in the pools within reserves, leading to a three-times higher accumulation of biomass (123.15 ± 25.81 g/m2 vs 42.26 ± 23.28 g/m2 in Grazed pools) and to lower nutrient export [1,40]. However, the influence of local factors independent from grazing cannot be totally ruled out.
4.1 Impact of grazing at the regional scale
The regional analysis shows differences in the species composition of the vegetation in the 16 studied pools. These differences are explained by grazing (14.1%), hydrology (9.9%) and soil characteristics (pH and phosphorus: 19.6%). Almost 30% of the species found in the 16 pools were exclusive to Ungrazed pools, while 15% were exclusive to Grazed ones and 55% common to both.
Grazing affects differentially the species and their abundance in the pools (Fig. 1) and most of species were consistently less abundant in Grazed pools than in Ungrazed ones due to the direct impact of herbivores (grazing and trampling). This notably concerns the species less tolerant to disturbance and the most palatable to livestock [26], which were more abundant in Ungrazed pools, i.e. located within hunting reserves (e.g., Juncus bufonius, Poa annua, Myosotis sicula, Phalaris caerulescens, Anthoxantum odoratum; Fig. 1). Conversely, the species that were more abundant in Grazed pools (Fig. 1) possess specific traits such as thorns (Carlina racemosa), hairiness (Hypericum tomentosum), repellent (i.e. aromatic) molecules (Glycyrrhiza foetida) or prostrate growth form (Plantago coronopus) [12,41] which make them little or ungrazed. Because these species are known to escape grazing, their abundance is used for assessment of grazing pressure [12,42].
The analysis of the impact of grazing on Pool species and Opportunistic species showed different responses of these groups to grazing. Pool species were favoured by the absence of grazing in contrast to Opportunistic and Rare species. The mechanisms involved are not clearly identified but could include weaker defences against herbivores for Pool species and/or the indirect impact of grazing on aquatic vegetation (through higher mechanical disturbance of submersed sediment, impact on water transparency…) [7]. No significant effect of grazing on the species richness per pool was found, nor on the number of Annuals, Perennials, Pool and Opportunistic species. This result contrasts with the presence of 2-times more exclusive species in Ungrazed (47 species) than in Grazed (23 species) pools. The number of species was not correlated with the size of the pools, which can probably be explained by various factors such as the diversity of micro-habitats, the shape of the depression or the intensity of competition. The vegetation density was low even in the Ungrazed pools, and no negative correlation was found between species richness and biomass, suggesting a weak intensity of competition. The density of species varies significantly along topographic (hydromorphic) gradients, but the analysis of the species richness at the pool level could not integrate that factor. The surface area is unlikely to play a major role in the species richness of pools [43].
4.2 Impact of grazing at the local scale (intra-community)
At the local scale (intra-pool), grazing had a lower but significant influence on species richness (Table 4) than topography (belts) and time (which explains mainly inter-annual hydrological variations). The impact of grazing on species richness (Total, Annuals and Perennials) was only significant in 2009, which was a wet year. Grazing thus interacts with hydrology, which had a smaller effect on the species richness in 2009 (significant only for the Total species richness and number of Annual species) (Table 3). The combined effect of hydrology and topography is usually the main variable that explains the species richness and the distribution of plant species in wetlands [25,44,45], the number of species declining when hydromorphy increases.
The effect of grazing was stronger on species abundance particularly that of Annuals (Table 4). The effect of grazing on the abundance of Perennials per quadrat showed a significant interaction between the grazing regime and years (Table 4) while the effect of year was not significant. In contrast, the impact of years on the abundance of annuals was much higher than that of grazing. These results show a stronger impact of grazing than of years (contrasted in terms of hydrology) or belts on the abundance of Perennials probably resulting from their long exposure to grazing. The long exposure of Perennial species to grazing and the restriction of the production by drought stress during the dry Mediterranean summers strongly affect their competitive ability [46]. However, the role of competition in the structure of plant communities is probably weak under dry Mediterranean climate. This was shown by the positive correlation found for 2008 between the abundance of Perennial species and the number of Annual species, as well as with the Total species richness. Similarly, the positive correlation between biomass and species richness (Fig. 2) suggests a limited role of competition in dry years even in the absence of grazing (pools within reserve). This highlights the role of abiotic factors, notably climate, in the control of the dominance of species [19–21] and thus in creating regeneration niches within plant communities that can be occupied by a large number of Annuals. In 2009 (wet year), the increased productivity changed the competitive relationships; this explains the non-significant correlation between the richness in Annuals and Perennials. No evidence of a potential reduction of the species richness by submersion stress or by higher production was found. This year corresponded probably for the plant community of the temporary pools to an intermediate disturbance (“Humped-back” model [47]). During dry years, the species richness was restricted by the low productivity.
5 Conclusions
Our results show a clear impact of grazing on the vegetation of the temporary pools of western Morocco at the pool scale and at the community scale. This effect is revealed at the pool scale by a decrease in species abundance, particularly the Pool species, but no effect is found on the Total species richness per pool. At the community scale, grazing leads to a decrease in species richness per quadrat, as well in species abundance; this impact is however lower than the one of hydrology. After 30 years of reserve management, no negative effect of the exclusion of grazing on species richness has been identified, as it is often found in European Mediterranean temporary pools [48,49]. The lack of negative effect is explained by the low productivity combined with the intensity of summer drought, which prevent the development of high biomasses, the establishment of woody species and helophytes, and generally limit the intensity of competition.
The weak impact of grazing found in this study on the species richness of pools contrasts with general hypotheses on the role of grazing in the disappearance or decline of species in Moroccan pools. This role is difficult to prove given the scarcity of Ungrazed pools and of related studies. The weak effect of grazing on the species richness of pools highlighted in this study should be carefully considered, taking into consideration that grazing exclusion was relatively recent (30 years). A weak effect imposed on longer periods of time may expose some species to an increased risk of local extinction by reducing their populations. Moreover, it is probable that seed production is higher in Ungrazed pools, due to higher peak biomass and to the correlation generally found between annual biomass and reproductive effort [50,51]. This higher production of seeds is likely to allow a greater resilience of population. Only species tolerant to grazing and disturbance could be able to remain in pools. More important datasets and long-term monitoring would be necessary for a better assessment of the impact of grazing on the density of seed stocks and on the demography of rare species, in the perspective of the conservation of Moroccan temporary pools.
Acknowledgments
We thank Florence Daubigney (TDV) and Mohammed Tellal (CRF, Rabat) for their logistical and technical support, Rachid Cheddadi, Eric Ambert, Raphael Mathevet, Mohamed Ibn Tattou, Semia Bensaad, Amina Daoud-Bouattour, Zeineb Ghrabi and Paul Roiron for helpful discussions. We thank the anonymous reviewers for their remarks that improved the quality of this manuscript. This project has been achieved with the financial support of the Egide-CMIFM program (PHC Volubilis MA/07/172) and was partly funded by the Fondation Tour du Valat, Fondation MAVA, Circle MedCoDyn project and CMPTM project (08/TM 82).
Appendix A List of species encountered in the study with the abbreviation used in text and figures and their attributes.
| Abbreviation | Annual/Perennial | Pool/Opportunist | |
| Agrostis salmantica | Ag.sal | A | P |
| Aira cupaniana | Ai.cup | A | O |
| Ammi majus | Am.maj | A | O |
| Anagallis arvensis | An.arv | A | O |
| Anthoxantum odoratum | An.odo | A | O |
| Antirhinum orontium | An.oro | A | O |
| Apium inundatum a | Ap.inu | P | P |
| Aphanes microcarpa | Ap.mic | A | O |
| Asterolinon linum-stellatum | As.lin | A | O |
| Avena sterilis | Av.ste | A | O |
| Baldellia ranunculoides | Ba.ran | P | P |
| Bellis annua | Be.ann | A | O |
| Biscutella didyma | Bi.did | A | O |
| Brachypodium distachyum | Br.dis | A | O |
| Briza maxima | Br.max | A | O |
| Briza minor | Br.min | A | O |
| Bromus mollis | Br.mol | A | O |
| Callitriche brutia | Ca.bru | A | P |
| Capsella bursa-pastoris | Ca.bur | A | O |
| Carex divisa | Ca.div | P | P |
| Carex divulsa | Ca.divs | P | P |
| Cardamine hirsuta | Ca.hir | A | O |
| Carlina racemosa | Ca.rac | A | O |
| Callitriche truncata | Ca.tru | A | P |
| Centaurea calcitrapa | Ce.cal | A | O |
| Centranthus calcitrapae | Ce.cal | A | O |
| Cerastium glomeratum | Ce.glo | A | O |
| Centaurium maritimum | Ce.mar | A | O |
| Centaurium pulchellum | Ce.pul | A | O |
| Chenopodium murale | Ch.mur | A | O |
| Cicendia filiformis | Ci.fil | A | P |
| Cistus monspeliensis | Ci.mon | P | O |
| Cistus salviifolius | Ci.sal | P | O |
| Corrigiola littoralis | Co.lit | A | P |
| Crassula tillea | Cr.til | A | O |
| Crassula vaillantii | Cr.vai | A | P |
| Cynodon dactylon | Cy.dac | P | O |
| Cyperus longus | Cy.lon | P | P |
| Daucus carota | Da.car | A | O |
| Daucus crinitus | Da.cri | P | O |
| Damasonium stellatum | Da.ste | A | P |
| Diplotaxis catholica | Di.cat | A | O |
| Dipcadi serotinum | Di.ser | P | O |
| Echium plantagineum | Ec.pla | A | O |
| Elatine alsinastrum a | El.als | A | P |
| Elatine brochonii a | El.bro | A | P |
| Eleocharis palustris | El.pal | P | P |
| Eryngium atlanticum | Er.atl | A | P |
| Erodium cicutarium | Er.cic | A | O |
| Euphorbia exigua | Eu.exi | A | O |
| Euphorbia helioscopia | Eu.hel | A | O |
| Exaculum pusillum a | Ex.pus | A | P |
| Filago gallica | Fi.gal | A | O |
| Filago germanica | Fi.ger | A | O |
| Foeniculum vulgare | Fo.vul | P | O |
| Gaudinia fragilis | Ga.fra | A | O |
| Galium murale | Ga.mur | A | O |
| Geranium molle | Ge.mol | A | O |
| Glyceria fluitans | Gl.flu | A | P |
| Glycyrrhiza foetida | Gl.foe | P | P |
| Heliotropium supinum | He.sup | A | P |
| Hordeum murinum | Ho.mur | A | O |
| Hyparrhenia hirta | Hy.hir | P | O |
| Hypericum perfoliatum | Hy.per | P | O |
| Hypericum tomentosum | Hy.tom | P | P |
| Illecebrum verticillatum | Il.ver | A | P |
| Isolepis cernua | Is.cer | A | P |
| Isoetes histrix | Is.his | P | P |
| Isoetes velata a | Is.vel | P | P |
| Juncus bufonius | Ju.buf | A | P |
| Juncus capitatus | Ju.cap | A | P |
| Juncus pygmaeus | Ju.pyg | A | P |
| Juncus striatus | Ju.str | A | P |
| Juncus tenageia | Ju.ten | A | P |
| Lamarkia aurea | La.aur | A | O |
| Laurentia michelii | La.mic | A | P |
| Lathyrus sphaericus | La.sph | A | O |
| Lavandula stoechas | La.sto | P | O |
| Legousia falcata | Le.spp | A | O |
| Leontodon taraxacoides | Le.tar | A | O |
| Kickxia commutata | Ki.com | A | P |
| Limonium sinuatum | Li.sin | P | O |
| Linum usitatissimum | Li.usi | A | O |
| Lotus hispidus | Lo.his | A | P |
| Lolium rigidum | Lo.rig | A | O |
| Lythrum borysthenicum | Ly.bor | A | P |
| Lythrum hyssopifolia | Ly.hys | A | P |
| Lythrum portula | Ly.por | A | P |
| Lythrum thymifolia a | Ly.thy | A | P |
| Lythrum tribracteatum | Ly.tri | A | P |
| Malva hispanica | Ma.his | A | O |
| Marsilea strigosa | Ma.str | P | P |
| Melilotus indica | Me.ind | A | O |
| Medicago polymorpha | Me.pol | A | O |
| Mentha pulegium | Me.pul | P | P |
| Myriophyllum alterniflorum a | My.alt | A | P |
| Myosotis sicula | My.sic | A | P |
| Narcissus viridiflorus | Na.vir | P | O |
| Nitella opaca | Ni.opa | A | P |
| Nitella translucens | Ni.tra | A | P |
| Ornithopus compressus | Or.com | A | O |
| Ormenis mixta | Or.mix | A | O |
| Ormenis praecox | Or.pra | A | P |
| Ornithogalum umbellatum | Or.umb | P | O |
| Panicum repens | Pa.rep | P | P |
| Phalaris caerulescens | Ph.cae | P | P |
| Pistorinia breviflora | Pi.bre | A | O |
| Pilularia minuta a | Pi.min | P | P |
| Plantago coronopus | Pl.cor | A | O |
| Plantago lagopus | Pl.lag | A | O |
| Plantago lanceolata | Pl.lan | A | O |
| Plantago psyllium | Pl.psy | A | O |
| Poa annua | Po.ann | A | O |
| Polygonum aviculare | Po.avi | A | O |
| Polypogon monspeliensis | Po.mon | A | P |
| Polycarpon tetraphyllum | Po.tet | A | O |
| Pulicaria arabica | Pu.ara | P | P |
| Pulicaria odora | Pu.odo | P | O |
| Ranunculus baudotii | Ra.bau | A | P |
| Ranunculus hederaceus | Ra.hed | A | P |
| Ranunculus muricatus | Ra.mur | A | P |
| Ranunculus ophioglossifolius | Ra.oph | A | P |
| Raphanus raphanistrum | Ra.rap | A | O |
| Ranunculus sardous | Ra.sar | A | P |
| Rumex bucephalophorus | Ru.buc | A | O |
| Scorpiurus vermiculatus | Ru.cri | P | P |
| Rumex pulcher | Ru.pul | P | P |
| Sagina apetala | Sa.ape | A | O |
| Sanguisorba minor | Sa.min | P | O |
| Scherardia arvensis | Sc.arv | A | O |
| Scilla autumnalis | Sc.aut | P | O |
| Schoenoplectus lacustris | Sc.lac | P | P |
| Scolymus hispanica | Sc.his | P | O |
| Scirpoides holoshoenus | Sc.hol | P | P |
| Scirpus littoralis | Sc.lit | P | P |
| Scirpus maritimus | Sc.mar | P | P |
| Scorpiurus vermiculatus | Sc.ver | A | O |
| Senecio vulgaris | Se.vul | A | O |
| Sonchus asper | So.asp | A | O |
| Spergularia rubra | Sp.rub | A | O |
| Stachys arvensis | St.arv | A | O |
| Tolpis barbata | To.bar | A | O |
| Trifolium angustifolium | Tr.ang | A | O |
| Trifolium campestre | Tr.cam | A | O |
| Trifolium micranthum | Tr.mic | A | O |
| Trifolium resupinatum | Tr.res | A | O |
| Trifolium scabrum | Tr.sca | A | O |
| Trifolium lappaceum | Tr.lap | A | O |
| Trifolium stellatum | Tr.ste | A | O |
| Trifolium subterraneum | Tr.sub | A | O |
| Trifolium tomentosum | Tr.tom | A | O |
| Tuberaria guttata | Tu.gut | A | O |
| Verbena supina | Ve.sup | A | P |
| Vicia sativa | Vi.sat | A | O |
| Vulpia myuros | Vu.myu | A | O |
a Rare species in Morocco according to Fennane and Ibn Tattou 1998 [34].


