1 Introduction
The Mediterranean region includes diversified wetlands [1,2] with high ecological [3,4], economic and social values [5]. Temporary wetlands in particular house a rare and endangered flora, which lend them an especially high conservation value [6–9]. These contribute greatly to regional biodiversities, and both their importance and vulnerability helped to identify several hotspots [3,10,11]. Temporary wetlands, abundant mainly on the southern side of the Mediterranean, are rapidly declining [12–16] because of strong human pressure [17–21]. To ensure the durability of these unique and endangered ecosystems, several inventory, assessment and monitoring programs have been implemented on Mediterranean wetlands (e.g. [22–25]), and Maghreb countries have recently developed various wetland-conservation measures and initiatives (National Parks, Nature Reserves and Ramsar sites). However, only a few studies focus on evaluating the impact of these conservation measures. For instance, the MELMARINA project aimed to establish integrated hydrological and ecological monitoring of North African coastal lagoons [26], the CASSARINA project assessed the current state of twelve North African lakes by making biological inventories and reconstructing their recent dynamics [27], and the Ichkeul National Park undergoes a regular monitoring of its marsh and lake habitats [28–33].
In Tunisia, with the exception of Ichkeul National Park, only one semi-permanent lake, Majen Chitane, has been protected since 1993. The lake and adjacent peatland (Dar el Orbi) have been studied repeatedly since the 1950s [27,34–39], but no synthesis of these works has ever been published. The CASSARINA project emphasized the major interest of this site for conservation [27], but the earlier works of Pottier-Alapetite & Labbe [34] and Pottier-Alapetite [35] were not considered. Moreover, Majen Choucha, another Tunisian semi-permanent lake that is relatively similar to Majen Chitane [21,35], is not yet protected. A comparison of these two sites taken with the synthesis of previous studies provides a unique opportunity to make an historical evaluation of the conservation value of both wetlands and the pertinence of the current conservation measures [40].
In recent years, palaeoecological and historical data have been used increasingly for defining baseline conditions of ecosystems, in order to assess the amplitude of their natural variability, their naturalness, their fragility, and the conservation status of rare species [41–44]. The need to connect ecological, palaeoecological and historical data has been highlighted since the 1990s [45–51]. Such interdisciplinary approaches offer theoretical and applied perspectives: they are particularly pertinent for a better comprehension of ecosystem functioning and they can help to refine conservation management policies [45,50–52]. However, they are not often used and are often ignored by scientists and managers [53]. This paper documents local ecological changes of Majen Choucha and Majen Chitane by comparing ancient and recent phytosociological relevés and by using the palaeoecological data available in the literature to: (1) evaluate the changes having affected Majen Chitane and Majen Choucha Lakes, and (2) assess and discuss the current conservation management of Majen Chitane Lake. This work attempts to demonstrate the usefulness and the need of interconnecting palaeoecological, historical and modern data for the conservation of Mediterranean wetlands.
2 Study sites
The terms used for North Africa wetlands vary greatly, depending on regions and countries. The term daya, for example, specifically refers to a Moroccan temporary pool [9], whereas in Tunisia, garâa (or garâet) designates a lowland lake, and majen (alternatively madjen, megene, majin or Majel), a small mountain lake [54]. Adding to the variety of local terminologies, changes or mistakes about the names of certain sites are not rare in the literature, and may create further confusion. This has been true for the studies carried out on Majen Chitane Lake, which has been named Lac des Nénuphars [35,55], Majen el Orbi [39], and Gaâret Sejnane [38]. This last name is definitely wrong, since Garâa Sejenane is the large alluvial plain close to the city of Sejenane [16] (Fig. 1).
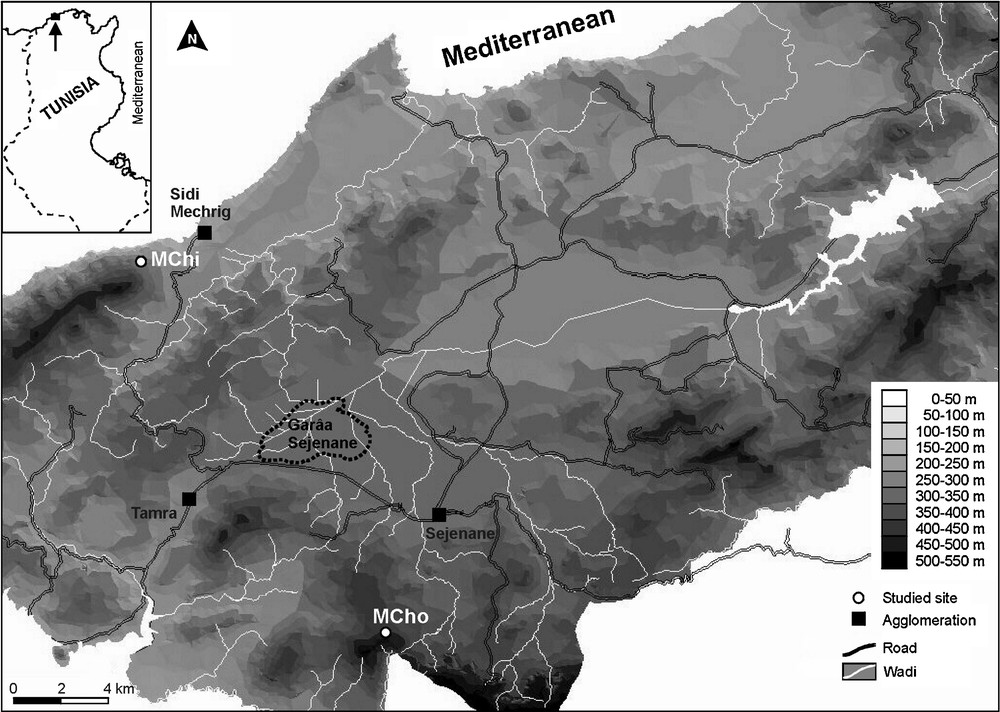
Location of the two studied semi-permanent lakes (Mogods region, northern Tunisia). MChi, Majen Chitane; MCho, Majen Choucha.
Majen Chitane and Majen Choucha Lakes (Fig. 1; Table 1) are located in the sandstone Oligocene Mogods Hills (northern Tunisia) and are separated by approximately 20 km. This region, with an annual rainfall between 800 to 1000 mm, is one of the rainiest in Tunisia, and has a Numidian flysh substratum [56]. It presents a Mediterranean wet bioclimate, with hot winters on the coast (Majen Chitane Lake), and mild winters inland (Majen Choucha Lake) [57]. The regional vegetation is dominated by degraded thermo-Mediterranean cork-oak forests and scrub, with Calicotome villosa, Erica arborea, Myrtus communis, Phillyrea latifolia, Pistacia lentiscus and Quercus coccifera.
Characteristics of the studied sites and dates of floristic surveys.
| Majen Chitane Lake | Majen Choucha Lake | |
| Code | MChi | MCho |
| Latitude N | 37° 09′ 10′′ | 37° 00′ 38′′ |
| Longitude E | 09° 05′ 53′′ | 09° 12′ 42′′ |
| Altitude (m) | 150 | 445 |
| Maximum depth (cm) | 185 | 100 |
| Area (ha) | 1.5 | 0.5 |
| Current conservation measure | Nature Reserve (1993) | |
| Ramsar site (2007) | None | |
| Date of phytosociological relevés | May 1951 [34,35] June 2010 (this study) | June 1951 [35] April 2008 (this study) |
| Date of floristic inventories | May, June, July 2006–2010 (this study) | February, April, May, July and December 2008–2011 (this study) |
Majen Chitane is a small (1.5 ha), semi-permanent (drying out during the driest years), freshwater, acidic, shallow (185 cm deep) lake located near the coast (37° 09′ 10′′N, 09° 05′ 53′′E; 150 m; Fig. 1) on a clayey depression of the northern slope of a sandstone relief called Jbel Chitane that rises to 454 m (Fig. 2A). It is fed by rainwater and by three small freshwater springs, two of which are permanent and trickle down through a Pteridium aquilinum peatland (Dar el Orbi; Fig. 2A) located 50 m above and almost fully cultivated (fruit trees, peppers, potatoes, tobacco…). The third stream does not cross the peatland and dries up in summer [58]. The springwater is pumped from late spring through summer to irrigate the cultivated part of the peatland. Since 1993, the lake and surrounding forest (5 ha) have been fenced and protected as a Nature Reserve. In 2007, the complex lake-peatland became a Ramsar site; however, the peatland, which is privately owned, is still cultivated today [59].

Semi-permanent freshwater acidic lakes in Tunisia. A, Majen Chitane Lake, largely covered by Nymphaea alba, overhung by the cultivated Dar el Orbi peatland (arrow) (S.D. Muller, July 2008). B, Majen Choucha Lake, surrounded by a grazed cork-oak forest (A. Ruhí, April 2010).
Majen Choucha is a similar, small (0.5 ha), shallow (100 cm deep) semi-permanent freshwater acidic lake located on the southern edge of Mogods Hills, on a clayey depression on the top of the sandstone massif of Jbel Choucha that rises to 513 m (37° 00′ 38′′N, 09° 12′ 42′′E; 445 m, Figs. 1 and 2B). Surrounded by an overgrazed cork-oak forest, the lake is isolated on the Jbel Choucha massif, and difficult to access, although cattle, sheep and goats regularly pass around it, and the local population uses it to gather the leeches that abound from spring to summer.
3 Materials and methods
3.1 Current vegetation
We undertook floristic inventories from 2006 to 2010 at Majen Chitane Lake and from 2008 to 2011 at Majen Choucha Lake. We constructed maps of the current vegetation for both lakes. Phytosociological relevés were carried out in the three vegetation belts (central, intermediate and marginal) of Majen Chitane (June 2010) and Majen Choucha (April 2008) Lakes using the Braun-Blanquet [60] method, with an abundance/dominance scale from + to 5. We compared them with data that had been collected on both lakes in May and June 1951 by Pottier-Alapetite and Labbe [34] and Pottier-Alapetite [35]. We distinguish below the temporary-pool species (amphibious species) characteristic of temporary habitats from the hydrophytic species (aquatic and helophytic species) characteristic of permanent waters. Plant nomenclature follows Le Floc’h et al. [61]. A correspondence analysis was performed (STATOS program, [62]) on the abundances of 62 species of the external belts (= intermediate + marginal belts) of Majen Chitane Lake (May 1951 and June 2010) and Majen Choucha Lake (June 1951 and April 2008). We excluded Myriophyllum alterniflorum, Schoenoplectus lacustris and Sparganium erectum, from this analysis because they are not included in the phytosociological relevés of Pottier-Alapetite and Labbe [34] and Pottier-Alapetite [35] while they were inventoried by these authors. We also excluded Nitella opaca and N. translucens (Charophytes), because these authors did not consider them.
3.2 Diachronic analysis of Majen Chitane
A spatial analysis was conducted from stereoscopic observation of aerial photographs (1948, 1963 and 1989; Office de la Topographie et de la Cartographie, Tunis), and a Google Earth image (2007; Google Earth, 2010). Maps of the vegetation in 1951 and 1997–98 maps were reported from [34,63]. The synthesis of previous palaeoecological studies [27,37–39,64–68] is used to reconstruct long-term local ecological dynamics.
4 Results
4.1 Vegetation
Phytosociological relevés and floristic inventories carried out in 1951 [30,31] and 2006-2011 (this study) in both lakes, identified 83 species (63 at Majen Chitane Lake and 55 at Majen Choucha Lake), among which 44.4% and 54.5% are hydrophytic and temporary-pool species, respectively (Table 2).
Floristic inventories and phytosociological relevés realized on the studied sites in 1951 [34,35], in 2006–2010 at Majen Chitane Lake (this study; May, June and July), and in 2008–2011 at Majen Choucha Lake (this study; February, April, May, July and December).
| Phytosociological relevés (external belt) | Floristic inventories | |||||||||||
| Majen Chitane | Majen Choucha | Majen Chitane | Majen Choucha | |||||||||
| Taxon | Synonym(s) | Code | Ecology | Statut | May 1951 | June 2010 | June 1951 | April 2008 | May 1951 | 2006–2010 | June 1951 | 2008–2011 |
| Agrostis pourretii Willd. | A. salmantica, A. pallida | Agpo | P | + | E | |||||||
| Alisma lanceolatum With. | A. plantago, A. plantago-aquatica | Alla | H | + | M | M,I | ||||||
| Alopecurus bulbosus Gouan | Albu | 1 | + | E | E | M | ||||||
| Anagallis arvensis L. | Anar | + | M | M | ||||||||
| Baldellia ranunculoides (L.) Parl.a | Alisma ranunculoides, Echinodorus ranunculoides | Bara | P | RR-NT | 1 | 1 | + | E | M,I | E | ||
| Bellis annua L. | M | |||||||||||
| Bellis prostrata Pomel | B. repens, B. radicans | Bepr | IF-NT | 1 | E | M | ||||||
| Bolboschoenus glaucus (Lam.) S.G. Smith | B. maritimus, Scirpus maritimus | Bogl | H | + | 1 | E | I | I | ||||
| Callitriche brutia Petagna | C. palustris subsp. pedunculata | Cabr | P | 1.2 | 1 | I,C | M,I | |||||
| Callitriche obtusangula Le Gall | Caob | H | 1 | 1 | + | 1 | E | M,I | E | M,I,C | ||
| Carex divulsa Stokes subsp. divulsa | Cadi | + | M | |||||||||
| Carex flacca Schreb. subsp. serrulata (Biv.) Greuter | C. flacca subsp. erythrostachys | Cafl | + | M | ||||||||
| Chara connivens Salzmann | H | RR | C | |||||||||
| Corrigiola litoralis L. | Coli | + | M | M | ||||||||
| Cotula coronopifolia L. | Coco | H | 2 | + | M,I | M | ||||||
| Cynodon dactylon (L.) Pers. | Cyda | 1 | + | M | E | M | ||||||
| Cynosurus polybracteatus Poir. | C. cristatus | Cypo | 1 | M | ||||||||
| Cyperus longus L. subsp. badius (Desf.) Bonnier & Layens | Cylo | H | 1 | M,I | ||||||||
| Damasonium bourgaei Coss. | D. alisma subsp. bourgaei | Dabo | P | + | M | |||||||
| Elatine alsinastrum L. | Elal | H | RR-CR | + | 1 | E | M,I,C | |||||
| Eleocharis multicaulis (Sm.) Desv. | Elmu | H | RR-NT | + | M | |||||||
| Eleocharis palustris (L.) Roem. & Schult. | Scirpus palustris | Elpa | H | 1 | 1 | E | M,I | M,I | ||||
| Eryngium pusillum L. | E. barrelieri | Erpu | P | 1 | + | + | E | E | M | |||
| Euphorbia chamaesyce L. | M | |||||||||||
| Exaculum pusillum (Lam.) Caruel | P | RR-NT | M | |||||||||
| Filago pygmaea L. | Evax pygmaea | M | ||||||||||
| Galactites tomentosa Moench | G. elegans | Gato | + | M | M | |||||||
| Galium palustre L. subsp. elongatum (C.Presl) Lange | G. elongatum | Gapa | H | + | + | E | M | |||||
| Glyceria spicata (Biv.) Guss. | G. fluitans subsp. spicata | Glsp | H | 1 | 1 | 2 | E | E-C | M,I,C | |||
| Helosciadium crassipes W.D.J. Koch ex Rchb. | Apium crassipes | Hecr | P | IF-NT | + | 4 | E-C | M,I | ||||
| Illecebrum verticillatum L. | Ilve | P | IF-LC | 1 | 3 | 1 | E | E | M,I | |||
| Isoetes velata A.Braun | Isve | P | IF-LC | 3 | 1 | + | 4 | E | M,I | E | M,I | |
| Isolepis cernua (Vahl.) Roemer & Schultes | Scirpus savii, S. cernuus | Isce | + | + | E | M | M | |||||
| Juncus acutus L. | Juac | 1 | M,I | |||||||||
| Juncus anceps Laharpe | Juan | 1 | M | |||||||||
| Juncus articulatus L. | Juar | H | 1 | M | ||||||||
| Juncus bufonius L. | J. bufonius subsp. eu-bufonius | Jubu | 1 | E | M | |||||||
| Juncus capitatus Weigel | Juca | + | E | |||||||||
| Juncus conglomeratus L. | Juco | 1 | M | |||||||||
| Juncus effusus L. | Juef | + | M | |||||||||
| Juncus heterophyllus Dufour | Juhe | H | 1 | + | 1 | C | I | E | M,I | |||
| Juncus pygmaeus Rich. ex Thuill. | Jupy | IF-LC | + | M | ||||||||
| Kickxia commutata (Reichenb.) Fritsch | Linaria commutata | Kico | P | 1 | M | |||||||
| Lotus angustissimus L. subsp. angustissimus | L. palustris | Loan | P | + | + | E | ||||||
| Lotus hispidus Desf. ex DC. | L. angustissimus subsp. hispidus | Lohi | 1 | + | M | M | ||||||
| Lythrum borysthenicum (Schrank) Litv. | L. nummularifolium, L. hispidulum | Lybo | P | R-LC | 1 | 1 | 1 | M | E | M | ||
| Lythrum hyssopifolia L. | Lyhy | 1 | 1 | + | E | M | E | M | ||||
| Lythrum junceum Banks & Sol. | Lyju | 1 | M | M | ||||||||
| Lythrum portula (L.) D.A. Webb | Peplis portula | Lypo | H | 1 | + | 1 | I | E | M,I | |||
| Lythrum tribracteatum Salzm. ex Spreng. | L. bibracteatum | P | R-LC | M | ||||||||
| Malva sylvestris L. | M | |||||||||||
| Mentha pulegium L. | Mepu | + | 1 | 1 | + | E | M | E | M | |||
| Myosotis sicula Guss. | M. lingulata var. sicula | Mysi | P | R-NT | 3 | + | 1 | E | E | M | ||
| Myriophyllum alterniflorum DC. | P | RR-LC | 1 | C | C | I,C | ||||||
| Nitella opaca (C.Agardh ex Bruzelius) C.Agardh | P | IF | 1 | M,I | ||||||||
| Nitella translucens (Persoon) C.Agardh | H | IF | + | M,I,C | ||||||||
| Nymphaea alba L. | H | R-VU | C | C | ||||||||
| Ornithopus compressus L. | Orco | + | M | |||||||||
| Panicum repens L. | Pare | 2.3 | 1.2 | M | M | |||||||
| Plantago coronopus L. | Plco | + | M | M | ||||||||
| Plantago lanceolata L. | Plla | + | M | |||||||||
| Poa annua L. | Poan | + | M | |||||||||
| Poa trivialis L. | Potr | + | E | |||||||||
| Polypogon monspeliensis (L.) Desf. | Pomo | + | M | |||||||||
| Portulaca oleracea L. | M | |||||||||||
| Ranunculus baudotii Godr. | R. aquatilis subsp. baudotii, R. peltatus | Raba | H | + | 3 | E | M,I,C | |||||
| Ranunculus muricatus L. | Ramu | + | 1 | E | M | |||||||
| Ranunculus ophioglossifolius Vill. | Paop | P | IF-LC | 1 | E | M | ||||||
| Ranunculus sardous Crantz. | R. sardous subsp. intermedius | Rasa | + | + | + | E | E | M | ||||
| Rumex pulcher L. | Rupu | + | M | |||||||||
| Sagina apetala Ard. | M | |||||||||||
| Schoenoplectus lacustris (L.) Palla | Scirpus lacustris | H | 1.2 | C | M,I,C | C | ||||||
| Silene laeta (Aiton) Godr. | Lychnis laeta | Sila | + | E | M | |||||||
| Solanum nigrum L. | M | |||||||||||
| Solenopsis laurentia (L.) C.Presl. | Laurentia michelii | Sola | P | + | + | E | E | M | ||||
| Sparganium erectum L. subsp. polyedrum Asch. & Graebn. | H | R-NT | 1.2 | C | M,I,C | |||||||
| Tamarix africana Poir. | Taaf | 1 | M,I | |||||||||
| Tolpis barbata (L.) Gartn. | Toba | + | M | |||||||||
| Trifolium campestre Schreb. | M | |||||||||||
| Trifolium filiforme L. | M | |||||||||||
| Trifolium resupinatum L. | M | |||||||||||
| Trifolium subterraneum L. | M | |||||||||||
| Typha domingensis (Pers.) Poir. ex Steud. | H | C |
a The two subspecies of Baldellia ranunculoides (subsp. ranunculoides and subsp. repens) were observed at Majen Chitane Lake, with a similar coverage.
Today, Majen Chitane Lake (Fig. 3 C) shows three belts: a central belt densely covered by Nymphaea alba except in the deepest water, an intermediate belt absent in the southern part of the lake and dominated by high helophytes (Bolboschoenus glaucus, Schoenoplectus lacustris and Sparganium erectum subsp. polyedrum), and a marginal discontinuous belt with rushes (Juncus acutus, J. conglomeratus and J. effusus) and some scattered amphibious species including Baldellia ranunculoides subsp. ranunculoides and subsp. repens, Cotula coronopifolia, Isoetes velata and Panicum repens. Majen Choucha Lake (Fig. 3D) is entirely covered by low vegetation structured in three distinct concentric belts: a central zone dominated by Callitriche obtusangula, Elatine alsinastrum and Myriophyllum alterniflorum, an intermediate zone dominated by Glyceria spicata, Isoetes velata and Ranunculus baudotii, and a marginal zone dominated by Helosciadium crassipes, Illecebrum verticillatum and Isoetes velata.
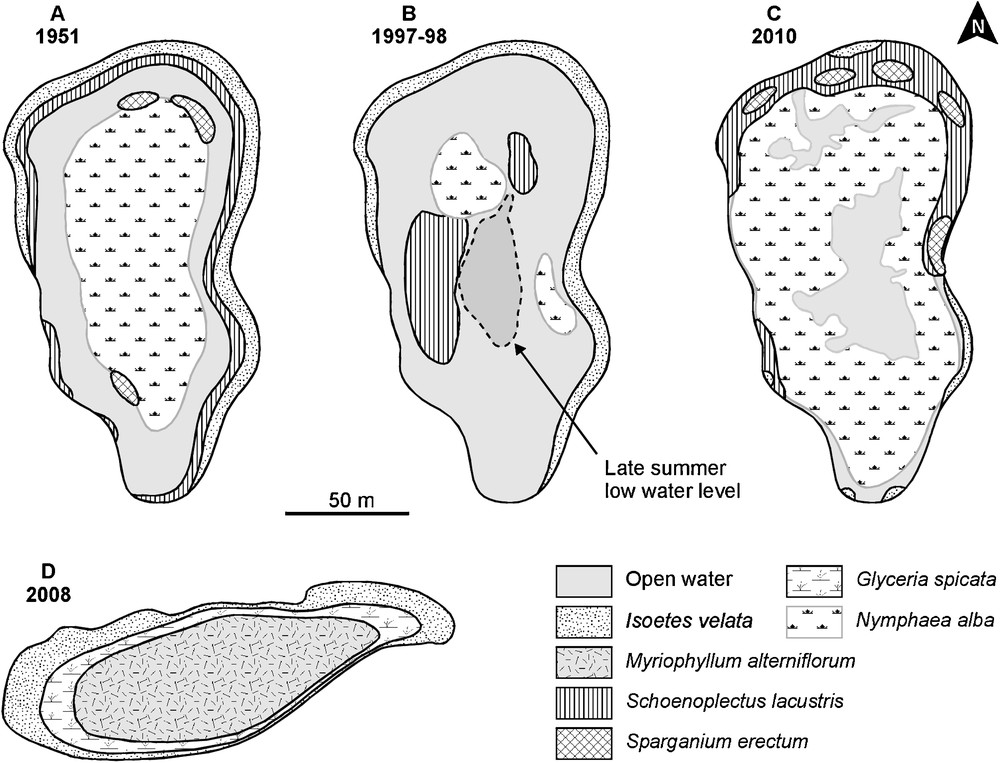
Vegetation maps of Majen Chitane Lake (A–C) and Majen Choucha Lake (D). A, May 1951 (modified from [34]); B, 1997–98 (modified from [63]); C, June 2010 (this study); D, April 2008 (this study).
The correspondence analysis (CA; Fig. 4) and floristic inventories carried out in 1951 and 2006–2011 (Table 2) reveal vegetation changes at both lakes between these two dates. Axis 1 contrasts the species of oligotrophic waters (Elatine alsinastrum, Illecebrum verticillatum, Isoetes velata) with the species of eutrophic environments (Cotula coronopifolia, Juncus heterophyllus, Lythrum portula). Axis 2 contrasts temporary-pool plants (Baldellia ranunculoides, Eryngium pusillum, Myosotis sicula, Ranunculus ophioglossifolius) with hydrophytic species (Alisma lanceolatum, Elatine alsinastrum, Ranunculus baudotii). The centroid of Majen Chitane Lake shows a large displacement along the axis 1 between 1951 and 2010 (Fig. 4), highlighting significant vegetation changes: (1) the disappearance of 13 species (Alopecurus bulbosus, Bellis prostrata, Eryngium pusillum, Glyceria spicata, Illecebrum verticillatum, Juncus bufonius, J. capitatus, Myosotis sicula, Myriophyllum alterniflorum, Ranunculus baudotii, R. ophioglossifolius, R. sardous and Solenopsis laurentia), which covered between 25–50% of the external belt in 1951 [35], and (2) the appearance of 36 species not recorded in 1951 [35], with only 28% of hydrophytic and temporary-pool species that cover less than 25% of the lake (Table 2). By contrast, the centroids of Majen Choucha Lake in 1951 and 2008 are very close on the factorial plane (Fig. 4), with a dominance of aquatic or amphibious species characteristic of oligotrophic habitats (Elatine alsinastrum, Illecebrum verticillatum, Isoetes velata). The only significant change in vegetation at Majen Choucha Lake since 1951 is the disappearance of Schoenoplectus lacustris and probably of Baldellia ranunculoides (Table 2).
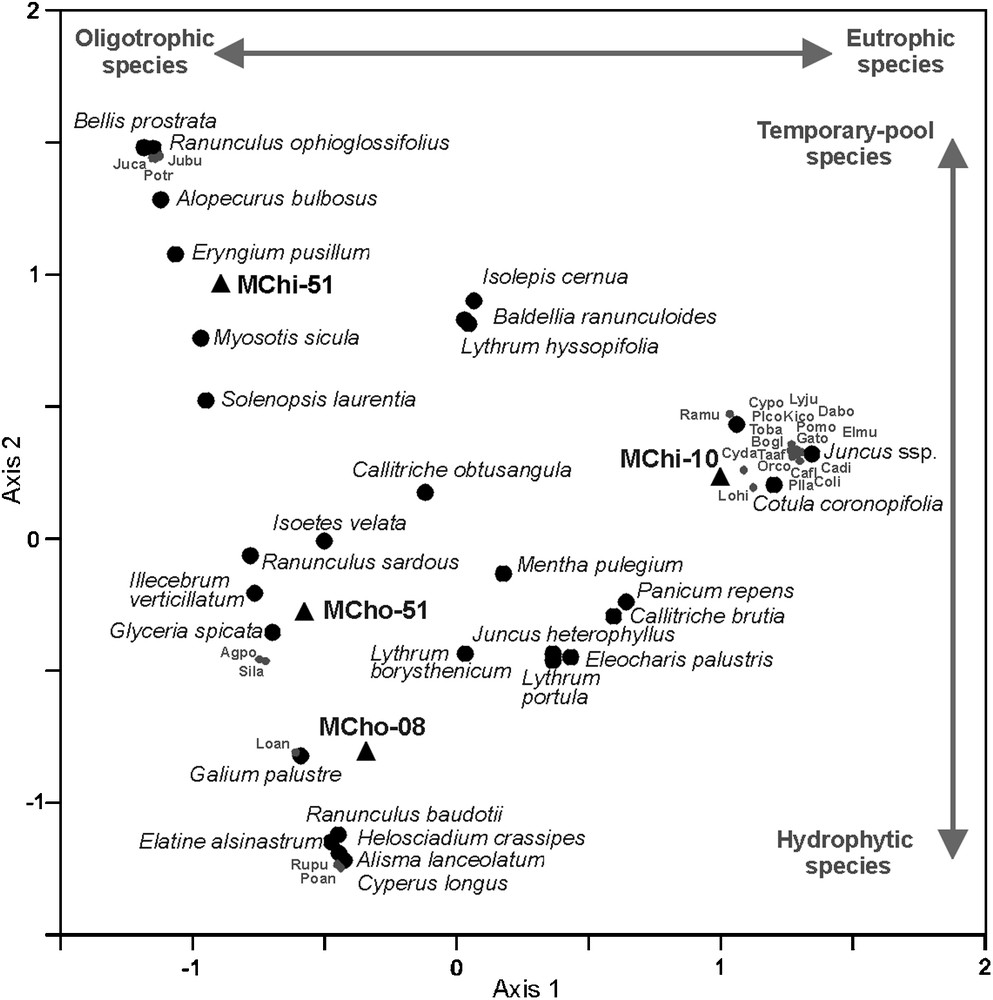
Scatterplot of the first two axis of correspondence analysis realized on species and phytosociological relevés carried out on the external belt (intermediate + marginal belts) of Majen Chitane Lake in May 1951 [35] (MChi-51) and June 2010 (this study; MChi-10), and of Majen Choucha Lake in June 1951 [35] (MCho-51) and April 2008 (this study; MCho-08). Inertia percentages of axis 1 and 2 are respectively 43.93 and 31.39. The signification of abbreviated species names is given Table 2.
The correspondence analysis (Fig. 4) and the floristic inventories done in 1951 and 2006–2011 (Table 2), show that Majen Chitane and Majen Choucha Lakes were very similar in the 1950s, harbouring a rich common plant cortege with respectively 63.0 and 70.8% of hydrophytic and temporary-pool species. Their common plant species were Alopecurus bulbosus, Baldellia ranunculoides, Callitriche obtusangula, Eryngium pusillum, Glyceria spicata, Illecebrum verticillatum, Isoetes velata, Juncus heterophyllus, Lythrum hyssopifolia, Mentha pulegium, Myosotis sicula, Myriophyllum alterniflorum, Ranunculus sardous, Schoenoplectus lacustris and Solenopsis laurentia. However, Majen Chitane Lake differed from Majen Choucha Lake in the occurrence of Bellis prostrata, Bolboschoenus glaucus, Nymphaea alba, Ranunculus ophioglossifolius and Sparganium erectum. Today, both lakes are very different (opposed centroids; Fig. 4): 22% of the hydrophytic and temporary-pool plant species have disappeared at Majen Chitane, against 14% at Majen Choucha.
4.2 Diachronic analysis of Majen Chitane
The stereoscopic observation of aerial photographs from 1948, 1963 and 1989, and the Google Earth image of 2007 (Fig. 5) reveal that the shape and size of Majen Chitane Lake have remained unchanged for more than 60 years. The August 2007 image shows the dense cover of Nymphaea alba. These images also show significant changes on the adjacent peatland Dar el Orbi: intact in 1948, its southern part was deforested and parceled in 1963, and entirely cultivated 1989. In 2007, the situation was similar except in the northwestern part where cultivation was abandoned. The last photograph also reveals the limits of the Nature Reserve around the lake, showing the regeneration of forest cover within the protected area. Moreover, ancient and modern vegetation maps (Fig. 3 [34,63]) of Majen Chitane Lake reveal strong changes since 1951: the major one is undoubtedly the decline of the amphibious community of Isoetes velata and Myosotis sicula (Myosotido siculae-Isoetetum velatae Pottier-Alapetite 1952), which constituted in 1951 a 2 m-wide belt on temporarily inundated shores along the three-quarters of the lake periphery [34,35]. This community comprised Alopecurus bulbosus, Baldellia ranunculoides, Bellis prostrata, Eryngium pusillum, Glyceria spicata, Illecebrum verticillatum, Isolepis cernua, Juncus bufonius, J. capitatus, Lythrum hyssopifolia, Poa trivialis, Ranunculus baudotii, R. ophioglossifolius, R. sardous, and Solenopsis laurentia (Table 2). The maps also show the decline of Schoenoplectus lacustris and Nymphaea alba (and the disappearance of Sparganium erectum?) in 1997–98, before their recent recovery.
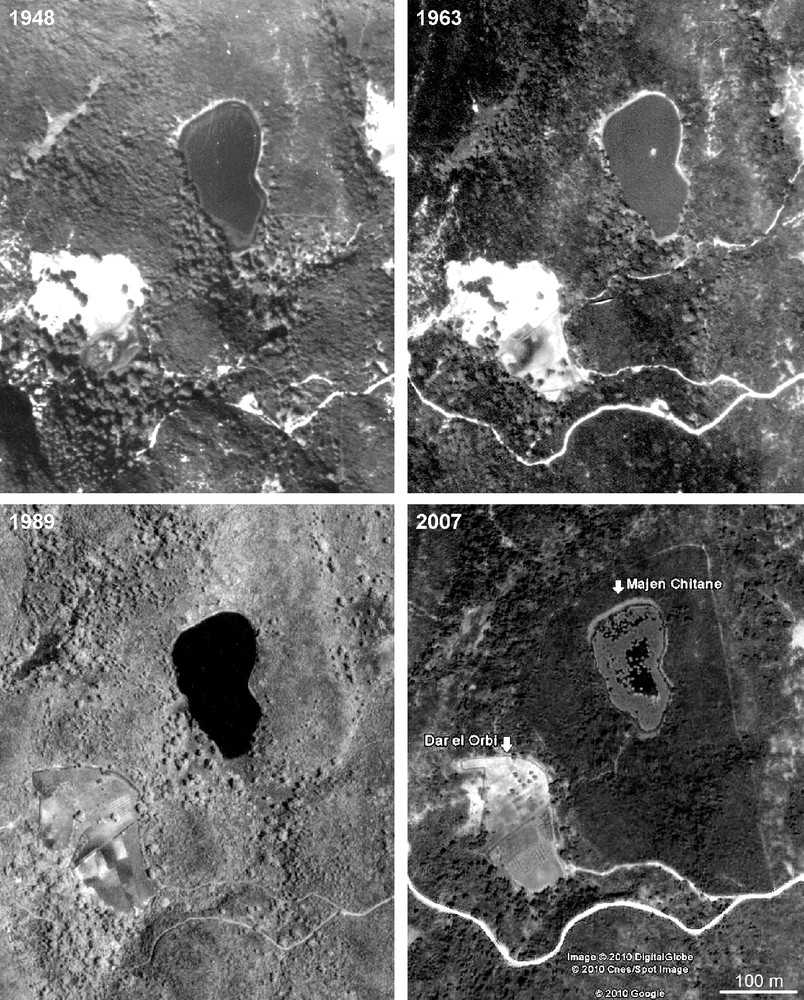
Aerial photographs of Majen Chitane Lake (right) and Dar el Orbi peatland (left); 1948, 1963, 1984 from Office de la Topographie et de la Cartographie, and 2007 from Google Earth (2010).
5 Discussion
5.1 Current state of the study sites
Despite the similarity of their locations, the present-day hydrophytic vegetation of the studied freshwater lakes is very different. Despite its smaller surface area, it is noteworthy that Majen Choucha Lake contains a higher proportion of hydrophytic and temporary-pool species than Majen Chitane Lake. This difference cannot be attributed to grazing, since livestock pressure has been shown to decrease the richness of hydrophytic and temporary-pool species [19] and grazing occurs around Majen Choucha Lake alone at the present. Majen Chitane Lake has highly turbid waters and abundant eutrophic species (Cotula coronopifolia, Juncus heterophyllus and Lythrum portula) on its southern shore, close to the spring inputs. These springs, which trickle through the cultivated peatland Dar el Orbi [63], probably leads to the lake enrichment in organic matter and its contamination by pesticides and fertilizers [58]. These features distinguish Majen Chitane Lake from Majen Choucha Lake, which presents clear and oligotrophic waters that allows the development of abundant populations of aquatic and amphibious plants: Elatine alsinastrum, Illecebrum verticillatum, Isoetes velata, Myriophyllum alterniflorum and Nitella opaca [9,69,70].
The present-day state of both lakes, as presented here, appears to be the result of human activities that are strongly different in each of the two sites: influence of close cultures for Majen Chitane and grazing for Majen Choucha. However, available present-day data are insufficient to definitely assess the role of human impacts and to distinguish them from the influence of physiographic parameters (surface area, water depth, altitude). Only a historical perspective is likely to confirm the relative role of anthropogenic and natural factors, by making it possible to reconstruct the baseline conditions prior to anthropogenic disturbances. The existence of historical [34,35,63] and palaeoecological records [37–39,64–68] at Majen Chitane Lake makes it possible to specify the mechanisms and consequences of past and present human activities, notably the peatland cultivation and the creation of the Nature Reserve.
5.2 Baseline ecological conditions
Two sediment cores were taken at the centre of Majen Chitane Lake in April 1997 for the CASSARINA project [37,64–68], and in summer 1999 [38,39]. A pollen diagram encompassing the last 5–6 millennia was obtained from the sediment core collected in 1999 [38,39]. Its study provides a minimal age for the sedimentation process, but lack of data about Isoetes and Nymphaea means that it is not usable for reconstructing the past dynamics of local communities. It nevertheless demonstrates the local occurrence, for more than 5000 years, of Persicaria (Polygonum persicaria-type), Myriophyllum, Cyperaceae, Sparganium-Typha and Typha latifolia, and the past abundance of Myriophyllum from 600 to 1100 AD, of Persicaria from 1100 to 1700 AD, and of Sparganium-Typha for the last thousand years. The core sampling conducted in 1997 provides more specific information on local environmental conditions during the last century, through the analysis of microfossils (zooplankton, diatoms, pollen) and macrofossils [37,64–68]. Considering the relative taxonomic imprecision of palaeoecological data, it is possible to assess the richness of the lake until the late 1950s between 20 and 28 hydrophytic and temporary-pool plant species [37,65,67] with Alismataceae, Cyperaceae (including Bolboschoenus and Schoenoplectus), Callitriche, Juncus spp., Nymphaea alba, Persicaria, Potamogeton natans-type, Ranunculus baudotii-type (section batrachium), Sparganium-Typha, and the species of oligotrophic waters Isoetes velata, Myriophyllum alterniflorum and Nitella opaca. This Charophyte species, whose presence is attested from the early 20th century, had its period of optimal development in the mid-1940s [37]. Previously unknown in Tunisia and therefore considered by these authors as new for the country, this species has since been found in numerous temporary wetlands of Tunisia, Algeria and Morocco, including Majen Choucha Lake [19,21,71] (H. Zouaïdia, I. Soulié-Märsche, unpublished data). During the first half of the 20th century, zooplankton (Alona rectangula, Chydorus sphaericus and Cypria ophtalmica [68]) and diatom assemblages (Cocconeis placentula, Eunotia spp., Gomphonema angustata, Navicula pupula, Pinularia subcapitata [66]) were characteristic of oligotrophic clear waters.
The floristic inventories and phytosociological relevés conducted in May 1951 at Majen Chitane Lake [34,35] show almost all the taxa evidenced in the fossil records, except Persicaria, Potamogeton and Nitella opaca. This reveals that Majen Chitane harboured for millennia until the 1950s plant communities of acidic, clear and oligotrophic waters, dominated by Isoetes velata and Myosotis sicula, and comprising Eryngium pusillum, Illecebrum verticillatum, Myriophyllum alterniflorum¸ Ranunculus ophioglossifolius and Solenopsis laurentia.
Comparison with the floristic inventories and phytosociological relevés of June 1951 at Majen Choucha Lake [35] reveals the past similarity of both lakes: the zonation of the Majen Chitane vegetation was close to the current one of Majen Choucha Lake and comprised Myriophyllum alterniflorum in central zones, and well-developed Myosotido siculae-Isoetetum velatae in marginal belts [34]. The population of Nymphaea alba and the helophytic intermediate belt of Majen Chitane Lake constituted the main differences between the two lakes in the 1950s [34,35]. Majen Chitane Lake, three-times larger than Majen Choucha and nearly twice as deep as it, is less sensitive to hydroclimatic changes and therefore more favourable to Nymphaea alba and high helophytes. That feature is probably amplified by the lower altitude and coastal setting of Majen Chitane, which make it resemble the wetlands of El-Kala and Guerbès-Senhadja regions in Algerian Numidia [72–74]. Except these differences, the plant communities of the two lakes were very similar. Consequently, the current situation of Majen Choucha Lake may be considered as a modern analogue of the ecological baseline conditions of Majen Chitane Lake, prior to anthropogenic changes.
5.3 Changes at Majen Chitane since the 1960s
The first changes affecting Majen Chitane Lake were recorded in the mid-1960s [64], contemporaneously with the beginning of the cultivation of Dar el Orbi peatland [63]. They comprised the gradual disappearance of eleven taxa present in the ecological [34,35] and palaeoecological records [37,65,67], and representing 40–55% of the total number of hydrophytic and temporary-pool plants, mostly rare and patrimonial: Eryngium pusillum, Glyceria spicata, Illecebrum verticillatum, Myosotis sicula, Myriophyllum alterniflorum, Nitella opaca, Persicaria sp., Potamogeton sp., Ranunculus baudotii, R. ophioglossifolius and Solenopsis laurentia. The post mid-1960s is also marked by zooplankton communities’ changes suggesting the onset of eutrophic conditions and environmental disturbances, with notably the replacement of Graptoleberis testudinaria and Tanytarsus sp. by Alonella excisa, Ceriodaphnia dubia, Chydorus sphaericus, Diaphanosoma brachyurum and Plumatella sp. [68]. In addition, phytoplankton communities characteristic of oligotrophic conditions were replaced, first in the mid-1960s, by two diatoms indicators of brackish conditions (Achnanthes delicatula and Synedra acus), and later around 1970, by a group including the diatom indicator of eutrophication Cyclotella pseudostelligera [66]. The lake's physicochemical and biotic changes have certainly been exacerbated by the introduction of exotic fishes (mostly carps) in the 1960s, whose scales have been found in recent sediments [65]. In the late 1980s, the lake's eutrophication is highlighted by the expansion of the nitrophilous Lemna minor and Cotula coronopifolia [63,67]. In 1998, Isoetes velata still constituted an 8 m-wide marginal belt, but none of the species characteristic of the Isoetion [34,35] have been reported by the authors [63]. Our surveys, conducted between 2006 and 2010, show that the Isoetes velata population has strongly declined and is now reduced to a few isolated individuals scattered along the shore and observed only once in 2010 since 2006. Because aquatic and amphibious plants grow better in clear waters than in disturbed ones [70], the highly turbid waters of Majen Chitane may explain this decline: among the species noted by Pottier-Alapetite and Labbe [34] and Pottier-Alapetite [35], only the plants of wide ecological amplitude (Baldellia ranunculoides, Callitriche obtusangula, Isoetes velata, Isolepis cernua, Juncus heterophyllus, Lythrum hyssopifolia, Mentha pulegium and Ranunculus muricatus) were recovered, whereas species strictly characteristic of oligotrophic temporary pools (Eryngium pusillum, Illecebrum verticillatum, Myosotis sicula, Ranunculus ophioglossifolius and Solenopsis laurentia) have disappeared. In addition, the observation in 2006-2010 of several species not noted in 1951 (e.g. Cotula coronopifolia, Cynodon dactylon, J. articulatus, Lythrum portula, Malva sylvestris, Panicum repens, Portulaca oleracea, Solanum nigrum) could translate an increase in human activities around the site. The cultivation of the peatland and the introduction of fishes thus appear to have destabilized the ecological balance of the lake and induced the replacement of the initial oligotrophic communities by eutrophication-tolerant ones.
The second change which has affected Majen Chitane Lake is related to variations of water level. In this line, a sedimentary change, marked by the input of mineral matter (silts and sands) to the lake centre, reflects the occurrence of several summer dryings between the 1960s and the 1990s [38,63]. These water-level lowerings could be due in part to drier climatic conditions, but they were undoubtedly aggravated by increasing water pumping for crop irrigation in the peatland [65]. Repeated dryings of the lake are probably the cause of the local disappearance of Nymphaea alba from the lake between 1964–1968 (M.N. Seddik, pers. comm.). This species was reintroduced in 1995 from its last Tunisian population located at Wadi el Herka (Wadi el Berka), a few kilometers east of Majen Chitane Lake (M.N. Seddik, pers. comm.). In spring 1998, the cover of Nymphaea alba was still reduced [63], while today, after eight rainy years, Nymphaea alba has almost completely recovered the lake centre except in its deepest waters. Water-level lowerings could also have favoured the spread of rushes on the lake shore, and the complete drying-up of the lake in 1993 has triggered the extinction of the introduced fish population (M.N. Seddik, pers. comm.). Such water-level changes have probably amplified the influence of water eutrophication on the plant and animal communities of the lake.
5.4 Implications for conservation
5.4.1 Naturalness of the lakes
The compiled palaeoecological and historical data allow the definition of baseline ecological conditions for the two sites under study, here considered as their state prior to 1960. These baseline conditions do not correspond to a “natural” state [42], in the sense that the wetland plant communities, considered to be highly resilient [19,75], have been shaped throughout North Africa by several millennia of grazing [76]. Indeed, a moderate grazing pressure, often used as a management tool of plant diversity of wetlands [70,77], contributes to the opening of habitats [9,78,79], limits competitive plant growth, and facilitates the development of light-demanding pioneer species, such as most of the annual species of temporary pools [9,71,79,80]. Such baseline conditions clearly correspond to a historical equilibrium of vegetation and traditional extensive human activities, before recent disequilibria, due locally to agriculture (pollution, hydrological changes) and the introduction of exotic fishes. The stability of the plant communities of Majen Choucha Lake since the 1950s reflects a balance between the maintenance of grazing and the natural protection of the site because of its isolation. The local disappearance of Schoenoplectus lacustris could nevertheless indicate hydroclimatic changes and/or an increase in grazing pressure. However, external factors do not threaten the ecological integrity of the site at that time, as they do for Majen Chitane.
5.4.2 Conservation stakes
Freshwater acidic mountain lakes, like Majen Chitane and Majen Choucha, are very rare in North Africa, and strong human pressure, particularly pollution, drainage and intensive water pumping for crop irrigation progressively leads to their degradation and even their disappearance [12,13,16,54,81,82]. Palaeoecological and historical records from Majen Chitane Lake, along with the comparison with Majen Choucha Lake, provide a long-term basis for assessing the conservation stakes of both sites. A large part of the hydrophytic and temporary-pool species present at Majen Chitane before 1950 was not recovered between 2006 and 2010. The Isoetes velata population experienced a strong decline since the 1950s resulting in its almost complete disappearance in the 2000s. This recalls the Isoetes setacea extinction from the temporary pool of Grammont (Montpellier, southern France) [83] and its replacement by Typha angustifolia. A palaeoecological study [80] confirmed the anthropogenic cause of this local extinction through water eutrophication and changes in the hydrological regime. The situation is comparable at Majen Chitane Lake where the shores are partly invaded by high rushes not indicated by Pottier-Alapetite and Labbe [34] and Pottier-Alapetite [35]. Given the damages at Majen Chitane Lake, it seems clear, a posteriori, that the conservation stakes were always significant at this site and that its protection was entirely justified. Moreover, the comparison between Majen Chitane and Majen Choucha Lakes shows the uniqueness and the high patrimonial value of the second site, which appears as Tunisia's last well-preserved semi-permanent lake. Majen Choucha Lake still harbours an exceptional flora, notably comprising Elatine alsinastrum, a species classified as endangered on the IUCN Red List of North Africa [84] and restricted to this site in Tunisia. Another population, which was still abundant in the 1950s in the temporary pool of Majen el Ma (Kroumirie) [35,85], has now disappeared. Majen Choucha Lake also presents a rich and diversified fauna, including in particular Pleurodeles nebulosus (Guichenot 1850), an amphibian endemic to Tunisia and Algeria, and classified by the IUCN as vulnerable, as well as Discoglossus pictus (Otth 1837), Lestes spp. and an unidentified Hirudinidae.
5.4.3 Current conservation efficiency of Majen Chitane and future needs
Despite the protection and restoration measures of some Mediterranean wetlands, the quality of their habitat is generally declining [33,54,63,86]. Complementary information from palaeoecological, historical and modern data provides a relevant framework to assess the efficiency of Majen Chitane Lake protection as a Nature Reserve since 1993 and as a Ramsar site since 2007. The only significant conservation action occurring at Majen Chitane Lake was the reintroduction of Nymphaea alba in 1995, which has saved it from extinction in Tunisia. Indeed, only three white water lily populations, including the Majen Chitane Lake one, existed in the 1950s: the second one, located near the Garâa Sejenane [87,88], has disappeared [21], and the third one, in Wadi el Herka, will be destroyed by the next impoundment of a dam. However, despite the reintroduction of Nymphaea alba, the lake's declining richness indicates that the protection measures were clearly insufficient to stop the ongoing degradation of its oligotrophic water communities, which is now probably irreversible without other management initiatives.
The palaeoecological and historical data from Majen Chitane Lake confirm that agricultural activities neighbouring Mediterranean wetlands represent their major threat [89], and reveal that stopping the cultivation and protecting the peatland are unavoidable conditions for restoring the lake. Although very degraded, the peatland still harbours vestiges of its original vegetation (Isolepis cernua, Juncus bulbosus, J. heterophyllus, J. tenageia, Lythrum junceum, Pteridium aquilinum, Samolus valerandi) including some rare (Anagallis crassifolia) and endemic species (Solenopsis bicolor): protecting it would probably suffice to enable the natural regeneration of the vegetation. This complete protection, recommended in the Ramsar convention of the complex lake-peatland [59], can only be achieved by having the Tunisian government acquire the peatland. In addition, the restoration of Majen Chitane Lake could be undertaken by resowing material collected at Majen Choucha Lake. Indeed, germination tests, carried out from the seed banks of ancient temporary pools, showed that the seeds and spores of the characteristic species have disappeared or are non-viable after 15–30 years [9]. In this line and according to the high patrimonial value of its flora and fauna, Majen Choucha Lake requires to be managed. In particular, and while no direct threat has been identified, the region's economic development and probable future increase in grazing pressure could threaten the ecological integrity of the site. It is not desirable to completely suppress grazing, but controlling of the periods when the lake is accessible and creating artificial pools in its vicinity should reduce the livestock pressure.
6 Conclusion
This paper demonstrates that it is possible, using available palaeoecological and historical data and with less extensive complementary investigations, to identify threats and define protection issues for specific wetlands. Wetlands lend themselves particularly well to this kind of study, because they record their own history through sedimentation process, and because their sedimentary archives are often studied for palaeoenvironmental and/or palaeoclimatic reconstructions, whose reliability increases along with methodological developments [41]. The interpretation of these data, in terms of local ecological dynamics, is essential for designing long and mid-term conservation-management policies [49,52]. As examples, some palaeoecology-based studies allowed refining conservation approaches [90–92] or specifying the introduced/native status of particular species [93,94].
In the case of the semi-permanent lakes of Tunisia, the available palaeoecological and historical data make it possible to define baseline conditions for conservation management, based on the evaluation of their degree of naturalness (sensu [42]). Such data moreover make it possible to interpret these baseline conditions as a state of equilibrium between the ecosystem and grazing, and considering Majen Choucha Lake as a unique and endangered heritage of the past relationships between nature and society. Rational conservation management of such exceptional wetlands is urgently needed, and requires interactions between scientists and managers, and the involvement of local populations and public administrations [95].
Finally, although the present study exemplifies the usefulness of existing historical and palaeoecological data for conservation, it should be noted that the impact of these data is often limited since they are generally adapted to other issues. To refine the understanding of past dynamics for future management, we must develop more systematically palaeoecological studies for conservation issues.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
Financial support was provided by Égide-CMCU (PHC Utique 07G0908), CMPTM (08-TM 82), and U.R. biogéographie, climatologie appliquée et dynamique érosive, FLAHM, université de la Manouba. We thank Mohamed Néjib Seddik (Head of District Forestry, Béja) for invaluable information on Majen Chitane, Leila Seyeh (Veterinary, Sejenane) for fieldwork help, Deborah Glassman for language improvement, and an anonymous reviewer whose comments were greatly appreciated. This paper is contribution ISEM 2011-095.


