1 Introduction
Deliberate or accidental introduction of plants and animals in ecosystems is considered as one of the major sources of biodiversity change. The Convention on Biological Diversity [1] regards it as one of the main factors of loss of biodiversity across the globe. There is a fairly large number of published studies on ecosystem impacts of invasive plants (sensu [2]) even though only few studies show evidence of negative impacts of invasive plant species on ecosystems [3–6]. Further, there can be regional differences in the impact of exotic plants (i.e. studies on Impatiens glandulifera in Europe: negative effects in northeast of England [7] compared to negligible effects in Czech Republic [8]. Their impact could be a reduction of local diversity [4] and changes in community composition [5]. However, other studies seem to contradict these assertions in [2].
The aim of this article is to study the threat to vegetation ascribed to an invasive plant, the tree-of-heaven (Ailanthus altissima (Mill.) Swingle), in a forest where it has begun to invade protected areas. This Simaroubaceae is native to China and was introduced all around the world for ornamental purposes. It was first planted in Europe in the mid-18th century. Due to its fast expansion, it is already considered as invasive in the majority of countries in which it was introduced [9,10]. It is very tolerant of urban conditions (nutrient and water deficient soil, polluted air city) and it can even grow in sidewalk cracks, parking lots and roadsides [11] Thus, it is in full expansion in big cities [12,13]. It settles fast in agglomerations [14,15] along railways and roads, and invades more natural zones. For example, it was recorded in peri-urban forests [16] in Hungary and in Western USA [17]. Its presence is, however, also recorded in old undisturbed forests in the USA ([18,19]).
A. altissima develops quickly thanks to its double strategy of reproduction [20]. Firstly, its sexual reproduction leads to high production of winged fruits scattered by wind over long distances [18]. Secondly, it also reproduces vegetatively by vigorous root suckers growing from root buds [21]. These biological characteristics alone could explain the strong capacity of colonization on a larger scale of this species. At specific site, the dark shade generated by the numerous root suckers could help it to compete efficiently with other plant species. Furthermore, it also produces allelochemical phytotoxic compounds that are known to inhibit the growth of herbaceous seedlings under artificial conditions [22–26]. However, the mere presence of phytotoxins in plants did not prove the occurrence of allelopathy under natural conditions [23]. The first study that described the impact of A. altissima on natural vegetal communities was conducted in Mediterranean habitats [27]. It is clear that the invasion of A. altissima leads to a reduction in diversity and richness of native Mediterranean vegetation. In a northern American temperate forest, another study showed allelopathic effects of A. altissima on coexisting native tree species (Quercus rubra, Acer rubrum and Acer saccharum) exposed to real spatio-temporal variation of allelochemical production and activity. In these transplantation experiments, A. altissima seems to have important negative effects on seedling growth of American native tree species [28], but increases soil fertility in its neighbourhood [29]. To our knowledge, nothing about the effect of A. altissima has been undertaken considering the whole forest vegetation and its possible regional variations. Actually, we wonder if A. altissima is a threat to the whole understory vegetation.
In this study, we examine the potential impact of A. altissima on the understory vegetation in situ in a peri-urban forest of the Parisian region known to host significant biodiversity [30]. This allowed us to observe, for the first time, the impact of A. altissima on the forest flora. As the Fontainebleau Forest is listed as MAB reserve and is invaded by A. altissima at its periphery, it was necessary to know the exact distribution of this invasive species to find out how much this forest is threatened. Our objectives were to assess:
- • the distribution of A. altissima in the Fontainebleau forest to quantify its occurrence in different types of habitats, and particularly within the forest;
- • its potential effect on floristic indices (Indigeneity, Rarity and Richness) of the shrub and herbaceous layers;
- • its role in the potential change on floristic communities’ composition.
We aimed to provide managers with reliable data on the threats to the forest's biodiversity caused by A. altissima.
2 Methods
2.1 Study area
The study area is the Fontainebleau forest, an old peri-urban forest located 65 km south of Paris, which covers an area of approximately 280 km2. It is considered as a “biogeographical crossroad” [31] because the climate is oceanic with continental, Nordic and even Mediterranean trends (mean annual temperature: 9.88 °C and annual rainfall: 700 mm). The tree layer is largely composed of Scots pine (Pinus sylvestris), common and Durmast oak (Quercus robur and Q. petraea) and beech (Fagus sylvatica). These natural conditions have allowed the development of an exceptionally rich fauna and flora: there are 6800 species of animals including more than 5000 insects and 5700 species of plants. Recently (1998), the Fontainebleau Biosphere Reserve was the 365th Reserve to be created in the world and the 10th in France. It covers 700 km2, including the forest of Fontainebleau. In this forest, A. altissima was reported for the first time in 1925 [32].
2.2 Distribution of A. altissima in the Fontainebleau forest
For management reasons, the forest is divided in plots (or sectors). The presence of A. altissima was sought in the totality of each forest sector by both managers and the authors. The distribution of A. altissima in the Fontainebleau forest was also assessed for each sector of the forest from the data provided by the managers and field checks. Furthermore, we carried out a broader survey to complement and update these data. We also radiated from every site where A. altissima had been located to precisely define the limits of the stands, i.e. where no new individual could be found. For each site, we reported all the data for presence and recorded the type of habitats: inner woods, meadows, riverbanks, borders of forest adjoining gardens, rails and roadsides. For statistical reasons, habitats that hosted less than ten mature A. altissima were discarded from analysis, i.e. meadows and riverbanks. Only four habitat types were thus considered: borders of forest adjoining gardens (Gardens), borders of forest adjoining railways (Rails), borders of forest adjoining roadsides (Roads) and inner woods (Woods).
2.3 Floristic plots
The data were collected once, mid-April to mid-July 2006. No significant differences emerged in terms of floristic richness between spring and summer (Kruskall-Wallis tests).
In each habitat (Table 1), we completed a floristic inventory (according to official French nomenclature [33]) under every A. altissima mature tree (i.e. sexually reproductive, and with a trunk diameter of about 30 cm). We recorded all present wild vascular species growing in a two-meter-radius circular plot centered on the tree trunk. Only non-overlapping plots were considered.
Number of plots inventoried under different tree species in each habitat type.
| Habitat types | ||||
| Tree species | Gardens | Rails | Roads | Woods |
| A. altissima | 31 | 47 | 79 | 30 |
| Acer pseudoplatanus | – | – | 15 | 1 |
| Carpinus betulus | 1 | – | 6 | 28 |
| Fagus sylvatica | 7 | – | 9 | 10 |
| Quercus petraea | – | – | 1 | 10 |
| Quercus robur | 13 | – | 18 | 18 |
To study the impact of A. altissima on the flora, we used three parameters: species richness (Richness), the total number of species observed in the site, Indigeneity, the proportion of indigenous (versus naturalized) species and Rarity. For a given species, rarity (Rasp) is the fraction of sites (including all habitats) in the department in which the species was not observed. Rasp varies between 0 (the species was observed in all sites) and 985/986 (the species was observed in a single site in the department). At the plot level, Rarity is the mean of specific rarity indices over all species observed in the plot.
To assess the impact of A. altissima on floristic indices, we also counted the number of its root suckers growing in the plots. Root suckers are easily recognized thanks to their particular morphology (large leaves, long internodes, brownish bark) [20]. Our aim was to explore the relationships between the number of suckers and the floristic indices in A. altissima plots.
For Gardens, Woods and Roads habitats, we studied an adjacent zone, for control purposes, where A. altissima was not present but with similar ecological conditions (shading and influences of anthropogenic factor) and 10 m distance from the invaded zones. For the Rail habitat, we were unable to find control zones in the neighbourhood of the invaded areas. In these “control zones”, we performed floristic inventories, with the same protocol, under the native mature tree species (Acer pseudoplatanus, Carpinus betulus, Fagus sylvatica, Quercus petraea and Quercus robur) (Table 1). Note that we did not perform enough vegetation inventories under Scots Pine to be able to use them statistically because the spatial distributions of these two species overlap very little in this forest. The effect of the different tree species’ morphology on understory vegetation was released by choosing trees whose trunks had approximately the same diameter as mature A. altissima. We compared understory vegetation of invaded plots (i.e. plots centered on A. altissima trunks) with that of control plots (i.e. centered on other tree species’ trunks). We recorded the species that had contrasting frequencies in the invaded vs control plots and compared their Landolt ecological indicator values (soil humidity, soil nitrogen content, pH and light, [34]).
2.4 Data analysis
Statistical analyses were performed using R 2.6.1 [35] and Minitab15.1.1.0 [36]. To describe the floristic diversity of the understory mature trees, we used classical indices [37]:
- • species richness (Rich) per plot;
- • indigeneity (Ind), i.e. the proportion of native (vs exotic) species within a plot (according to the regional list established by the National Botanical Conservatory of the Paris Basin, [38]);
- • average species rarity (Rar) per plot (species rarity given in terms of regional data, referring to the proportion of sites where a given species was not observed, across all habitats of the region [39]).
Two-way ANOVAs were performed to test for significant differences in the plots’ floristic indices:
- • among habitats;
- • among invaded vs control zones;
- • in terms of interaction.
Spearman tests assessed the relationships between root suckers density and floristic indices in invaded plots.
We used the distance-based redundancy analysis (dbRDA) [40] based on species presence/absence data to detect significant differences in the floristic composition of plots among habitat types and among invaded vs control plots in each habitat type. Finally, Wilcoxon non-parametric tests were used to compare the Landolt ecological values [34] of the species with contrasting frequencies in the invaded vs. control plots.
3 Results
In the Fontainebleau forest A. altissima was found mostly along borders of different habitats. Its distribution in 2006 is shown on the map of Fig. 1.
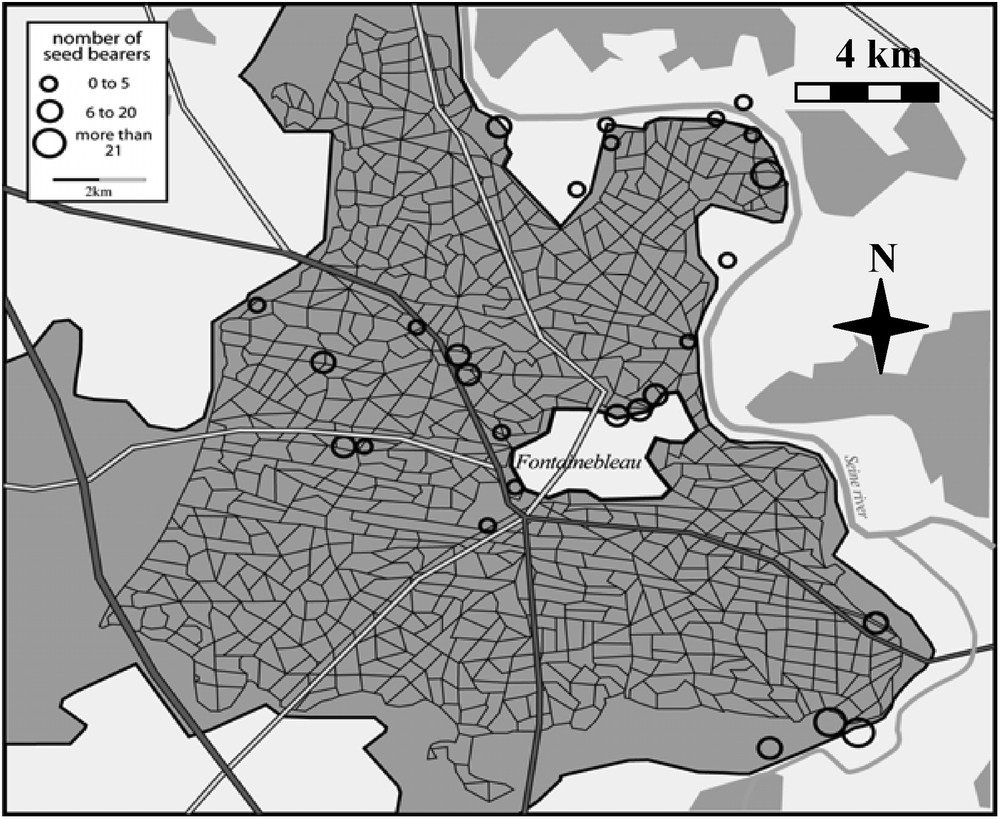
Map of the Fontainebleau forest showing all the A. altissima sites (circles).
We inventoried 324 plots, i.e. 187 invaded plots (at the base of A. altissima mature trees) and 137 control plots (at the base of native mature trees). We observed a total of 241 plant species, including 209 native species (86.7%).
3.1 Floristic index variations
The analysis of variance indicated the existence of significant differences for indigeneity and rarity among habitats, for richness and rarity among invaded vs. control plots, and for indigeneity in a cross analysis of the two factors (Table 2). No effect of habitat on richness was observed; we found an average of 9.1 species (SD = 3.2) per invaded plot and 12.4 species (SD = 3.8) per control plot. Mean species rarity was significantly lower in A. altissima plots (mean rarity = 0.88 and SD = 0.03 vs. 0.89 and 0.03 respectively in control plots) and in plots located in Gardens (0.87, SD = 0.02) than in those located in Woods and Roads (0.90, SD = 0.03 for both) (Fig. 2).
ANOVA for each floristic diversity index.
| Df | Richness | Rarity | Indigeneity | ||||
| F value | Pr (>F) | F value | Pr (>F) | F value | Pr (>F) | ||
| Habitat | 2 | 2.9 | 0.06 |
17 | 1.10E-07 | 6.5 | 1.70E-03 |
| Invasive (vs native) | 1 | 36.5 | 5.00E-09 | 7.7 | 0.01 | 2 | 0.16 |
| Habitat*Invasive | 2 | 2.5 | 0.08 | 0.6 | 0.55 | 3.5 | 0.03 |
| Residuals | 271 |
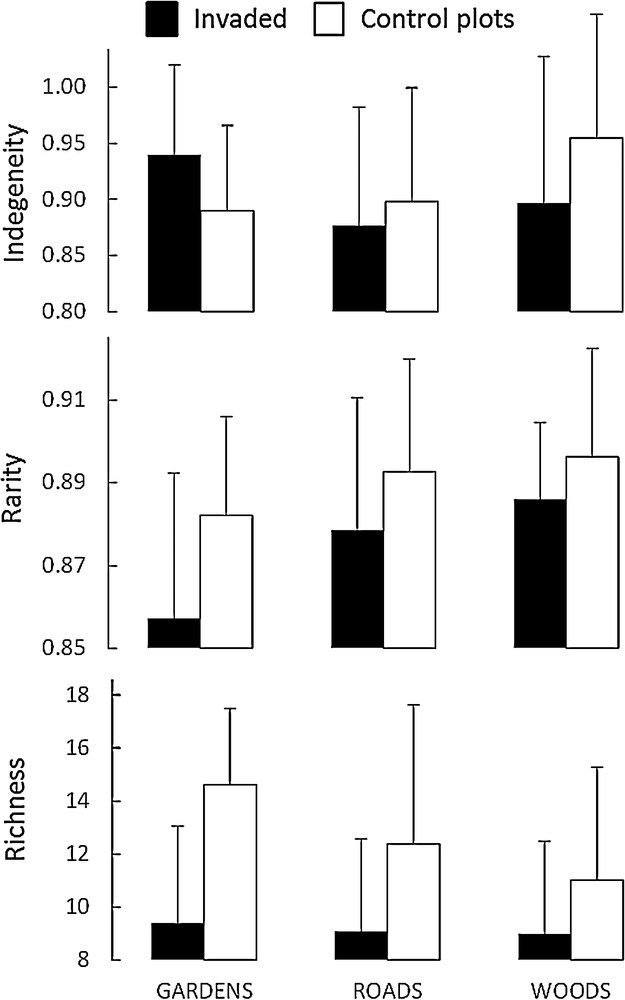
Mean values for invaded vs native plots across the three habitat types and for each floristic diversity index. Error bars correspond to standard deviation.
3.2 Relationships between root sucker density and floristic indices per plot
Within the invaded plots, the number of root suckers observed ranged from 0 to 88 and was negatively correlated with species richness under A. altissima (Rich = 9.43–0.06 × A. altissima root suckers, Spearman test: r2 = 0.11; P-value < 0.0001) (Fig. 3). In contrast, neither plot indigeneity nor plot rarity was significantly correlated with sucker density (results not shown).
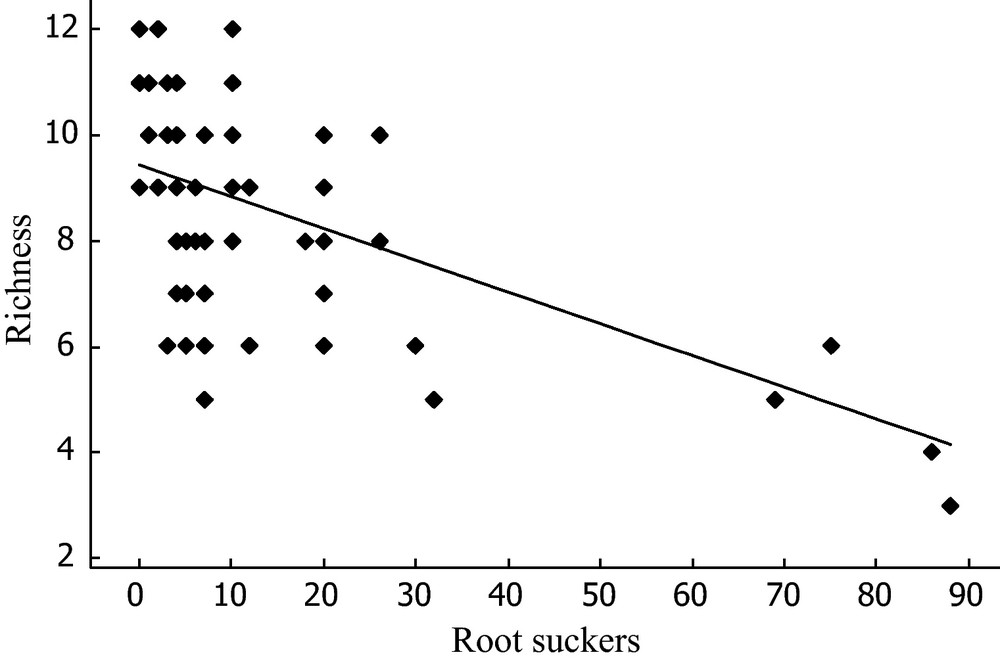
Relationships between root sucker number and specific richness in invaded plots.
3.3 Comparison of understory vegetation between invaded and control plots
In the inventories performed under A. altissima trees, 168 species were counted, whereas in the inventories performed under native trees, 193 species were recorded.
In each habitat type, we compared the floristic composition in A. altissima plots vs control plots. In Gardens, Roads and Woods species, composition differed significantly between invaded and control plots (dbRDA test: P-value < 0.01). Among the species the most frequently recorded under native trees, some were hardly observed under A. altissima, e.g. Brachypodium sylvaticum and Lonicera periclymenum (Table 3). In contrast, some common species recorded in more than 50% of A. altissima plots were less frequent under native trees, e.g. Galium aparine and Alliaria petiolata. The subspontaneous species Parthenocissus quinquefolia was only recorded under A. altissima. The Wilcoxon tests showed no significant difference in Landolt ecological values (Wilcoxon tests: Humidity P-value = 0.53, pH P-value = 1, Nitrogen P-value = 0.067 and Light P-value = 0.46) between these two classes of species. Nevertheless, the mean nitrogen value for the species mainly found under A. altissima is 4.0 (nitrogen rich), whereas those mostly found in control plots present a value of 3.2 (medium level of nitrogen) (Table 3).
Frequency of species and their Landolt ecological values [35], in control and invaded plots. Only species whose frequency differences were highly significant (Chi2 square tests not shown) and higher than 10% are listed.
| Species observed at the base of trees | Control plots (%) | Invaded plots (%) | Landolt ecological values | |||
| Humidity | pH | Nitrogen | Light | |||
| Galium aparine | 18 | 51 | 3 | 3 | 5 | 3 |
| Alliaria petiolata | 30 | 55 | 3 | 3 | 5 | 2 |
| Ulmus minor | 5 | 20 | 3 | 4 | 3 | 3 |
| Bromus sterilis | 3 | 16 | 2 | 3 | 4 | 3 |
| Parthenocissus quinquefolia | 0 | 13 | 3 | 3 | 3 | 3 |
| Veronica hederifolia | 4 | 16 | 3 | 3 | 4 | 3 |
| Brachypodium sylvaticum | 32 | 7 | 4 | 3 | 4 | 3 |
| Lonicera periclymenum | 28 | 4 | 3 | 2 | 3 | 3 |
| Rubus fruticosus gr. | 59 | 36 | 3 | 4 | 4 | 4 |
| Melica uniflora | 29 | 9 | 3 | 2 | 2 | 2 |
| Carpinus betulus | 31 | 11 | 3 | 3 | 3 | 2 |
| Galeopsis tetrahit | 22 | 3 | 3 | 3 | 5 | 3 |
| Fagus sylvatica | 20 | 3 | 3 | – | 3 | 1 |
| Milium effusum | 15 | 1 | 3 | 3 | 3 | 2 |
| Poa nemoralis | 15 | 1 | 2 | 3 | 3 | 3 |
| Euphorbia cyparissias | 15 | 2 | 2 | 3 | 2 | 4 |
| Acer campestre | 16 | 4 | 3 | 4 | 3 | 3 |
| Fraxinus excelsior | 20 | 9 | 4 | 4 | 4 | 2 |
| Polygonatum multiflorum | 12 | 1 | 3 | 3 | 3 | 2 |
| Rosa arvensis | 12 | 2 | 3 | 4 | 3 | 3 |
| Anemone ynemorosa | 16 | 5 | 3 | 3 | 3 | 2 |
4 Discussion
4.1 A. altissima distribution
Our fairly complete field survey of the Fontainebleau forest showed that A. altissima grows essentially in edge habitats or disturbed sites, i.e. along the edge of roads, railways, gardens, meadows and river banks. This result concurs with previous observations [18,19], who noticed that this species was mainly found in disturbed open areas like forest borders from which it can spread in the underwood, where light is not a limiting factor [41–43]. We presume the A. altissima we found within the forest stands correspond to individuals that were favoured by temporary gaps in the canopy at least 30 years ago when these forest stands were a garden, a tree nursery or a vegetatively restored sandpit that were abandoned since (according to the managers’ accounts). From that time, the trees produced a bank of root suckers and established durably forming a monospecific canopy preventing the regeneration of other tree species [20]. This species could thus be a threat particularly as open habitats or natural or anthropogenic gaps regularly occur in the canopy.
The preferred location of A. altissima along the lines of communication involves a special vigilance on the part of management of future outbreaks of this species in these areas for an early elimination of a potential new centre of invasion. Managers should also avoid slight thinning of forest area or at least ensure that revegetation is sufficiently rapid and complete to be sure that A. altissima will not install.
4.2 A. altissima and understory plant communities
The range of species able to grow in its neighbourhood is not especially low. The subtle differences in species composition could be explained by the fact that the study focused on the plants of the woods and edges, already adapted to competition for light, water and nutrients. A feature common to these species that persist under A. altissima, could be the resistance to herbicide allelopathic compounds produced by the leaves, bark and roots. The species most frequently observed were very common in the region, e.g. Hedera helix (146 plots, Rar = 0.78), Galium aparine (95 plots, Rar = 0.75), or at least in the forest areas of the region, e.g. Alliaria petiolata (103 plots, Rar = 0.95).
In every habitat type where we were able to compare diversity under A. altissima and native tree species in control zones, understory vegetation under A. altissima was significantly poorer and more common per plot than under native trees (Table 2). The effect of A. altissima on indigeneity is only detected in Woods plots. Two hypotheses could be given to explain those effects:
- • A. altissima spontaneously invades disturbed microsites hardly colonized by other species (passenger hypothesis [44]), e.g. in Roads and Gardens known as disturbed habitats;
- • the presence of A. altissima leads to the impoverishment of diversity through competition and allelopathy (driver hypothesis [44]), e.g. in Woods, a less disturbed habitat.
Our results seem to indicate that establishment of A. altissima is facilitated by disturbance but once the species is established, it persists, spreads and displaces native vegetation.
The loss of diversity under A. altissima amounts to 15 to 30% approximately, depending on which tree species they are compared with and which habitat they grow in. A similar loss was previously observed in Mediterranean islands [27], or in a disturbed forest of Virginia [45].
The factor that seems to influence the diversity of the understory is the density of A. altissima suckers. We found a negative correlation between their number and the species richness recorded in the plots (even if our sample did not reveal a real gradient of density). This effect could be attributed both to interspecific competition and to allelopathic properties of A. altissima. Indeed, the number of 88 suckers per plot refers to about seven or eight suckers per square meter. The growth of such vigorous organisms implies high water and nutrient consumption and thick shade [20], and therefore, the elimination of the most sensitive species. Yet, allelopathy should also be mentioned since it was previously highlighted by numerous authors: [23,46–48] in ex situ conditions. In a field experiment in a temperate forest, the growth of seedlings of Acer saccharum, A. rubrum and Q. rubra were also affected by presence of A. altissima in their neighbourhood [28]. On the scale of tree bases, allelopathic effects could be produced by the root system [49] or by leaching of the foliage [20]. A. altissima suckers produce greater amounts of inhibitory compounds than adult trees [50]. We can suppose that allelopathy is all the more efficient as the sucker density is high (Allee effect) [51,52].
In any case, the communities of species recorded under A. altissima were significantly different from those found under other tree species. This was confirmed in all habitats studied. We presume the chemicals it releases deeply modify the environmental conditions of its understory - giving rise to floristic communities characterized by resistant populations or indifferent species to theses substances. For example, populations of exotic Parthenocissus quinquefolia are only recorded under tree-of-heaven. A. altissima is a very new species in Europe, and thus in this forest. We suppose it could inhibit the growth of species not adapting to its allelopathic properties, e.g. Millium effusum, Poa nemoralis and Polygonatum multiflorum, which we found relatively frequently under native trees but almost never under A. altissima. These three herbaceous plants are indeed known to be sensible to species composition of canopy trees. Millium effusum and Polygonatum multiflorum are vulnerable to compounds produced by Fagus sylvatica [53,54] whereas Poa nemoralis by Quercus cerris and Q. conferta [55].
The control plots were chosen in sites that are ecologically similar to the invaded areas and thus we did not really choose the native species we studied. Consequently, we often compared species composition in the understory of a variable number of native tree species. This induces certainly a bias in the results but our choice was to favour an ecological homogeneity among sites we compared.
Our results indeed showed that the species that mainly differed in frequency, between invaded and control plots, had the same ecological needs, with the exception of the soil's nitrogen contents that could be higher in invaded plots [29,56]. The non-significant result in Landolt ecological values could indeed be due to the low number of species taken into account (test power).
For these few species that are favoured in invaded plots (i.e. Galium aparine, Alliaria petiolata, Bromus sterilis and Veronica hederifolia) A. altissima could be a facilitator. There is a significant positive interaction and clear ecological significance between nitrogen in Galium aparine and in Alliaria petiolata [57] as for Bromus sterilis [58]. It has not been demonstrated so clearly that Veronica hederifolia was favoured by nitrogen [59].
5 Conclusion
In this study, we showed that A. altissima was present in the Fontainebleau forest, not only along roads and railways but also penetrating the forest due to natural or artificial gaps. Within the forest patches, once this species has invaded, it seems to settle in the long term. That settlement prevents the growth of a large number of other plant species and modifies the structure of the community through facilitation for common nitrophilous species and competition and allelopathy against sensitive forest species. In this way, in some areas of the Fontainebleau forest, A. altissima directly threatens flora of protected reserves. The data of this study will thus be taken into account in management plans to strictly limit its expansion in the forest.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We especially thank Monika Zavodna and Claire Jousseau for comments on drafts of this paper, Steve Hubert for the graphics. Claude Lagarde of the Office National des Forêts (ONF) provided helpful information. The English correction was performed by Anne Lindsey.


