1 Introduction
In spite of a number of works carried out these last decades, the mechanism of proteinuria remains controversial. The most important data recently obtained with different methods such as isolated perfused kidney, micropuncture of single nephrons, tissues uptake techniques, isolated glomerular cells, immunofluorescence or genetic studies, have been presented and discussed in different reviews, some of them focused on the intricate properties of the glomerular filtration barrier (GFB) [1,2], other on the cell biology of the podocyte [3], the GFB structure [4] or on the genetic aspect in human disease [5,6]. Even if more attention is now drawn to the responsibility of the podocyte, most of the current interpretations remain focused on a defect of the GFB permeability, which is still considered as the prime cause of human proteinuria. In this approach, the ultrastructural changes observed in both the cytoplasm and the nuclei of the podocytes of proteinuric patients seemed to be underestimated.
In this work, a more complete analysis of both the intracytoplasmic and intranuclear changes in the podocytes of three severe nephrotic syndromes has been carried out, using a conventional transmission electron microscope (TEM) and a high resolution scanning ion microscope (SIM).
2 Material and methods
2.1 Patients
Renal biopsies of five patients have been studied by both electron and ion microscopy. Three of these patients had a severe nephrotic syndrome (NS), with high proteinuria at the time of the biopsy, and two, only a microscopic hematuria without any proteinuria.
Case 1: Eight months old child at the time of the biopsy. Very severe NS. Proteinuria > 4 g/day. Light microscopy (LM): minimal glomerular changes (MGC) in 20 glomeruli. Immuno fluorescence (IF): IgG, IgA, C4 and C3 in a number of tubular “reabsorption” droplets.
Case 2: Five years old at the time of the biopsy. Proteinuria: 4.2 g/day. Steroid resistant NS (SRNS). LM: MGC in 50 glomeruly. IF: IGM (trace in the mesangium).
Case 3: 18 months old at the time of the biopsy. Proteinuria: 5.9 g/day. SRNS. LM: MGC in 25 glomeruli.
Cases 4 (eight years old) and 5 (four years old): only microscopic hematuria without proteinuria. LM: no glomerular change.
2.2 Tissue preparation and instrumentation
All biopsies were fixed in gluteraldehyde, post fixed in osmium tetroxyde, and embedded in epon using the traditional methods of TEM. Ultra thin (∼50 nm) sections, deposited on copper grids, were studied, with or without additional contrast, using a TEM equipped with a CCD camera (Morgagni 268-D).
For SIM, semi-thin (∼1 μm) sections were deposited on ultra pure gold or silicon plates, and a high resolution SIM (CAMECA nanoSIMS 50) was used. This instrument allows analytical images, representing the distributions of isotopes (stable or radioactive) present at the surface of the specimen, to be obtained with a spatial resolution which, at best, is of the order of 50 nm. Briefly summarized here, with this instrument, a very thin ion beam, formed of cesium ions, called primary ions (PI) is focused onto the surface of the specimen, on an area typically of the order of 50–100 nm in diameter. As a result of this bombardment, the atoms of the surface of the specimen are ejected, and a sub microscopic crater is formed, the diameter of which being the diameter of the impact of the PI beam, and the depth, typically of the order of several atomic layers (depending on the duration of the impact and on the intensity of the PI beam). From the crater, the pulverized atoms (or groups of atoms) are emitted either as neutral or charged particles (positive or negative). The charged particles called secondary ions (SI), characteristic of the crater, are collected, analyzed with a mass spectrometer and selected according to their mass/charge ratio. By scanning point per point the surface of the specimen, after selection of one variety of SI, the chemical image of the selected charged particle is obtained. For more details concerning the principle of ion microscopy, also called secondary ion mass spectrometry (SIMS) and its applications to the study of biological tissues, the reader is referred to previous reviews [7–10].
In this work, the distributions of three atoms, nitrogen (N), phosphorus (P) and sulfur (S) have been studied. The negative mono atomic SI, P–and S–, have been used for the study of the distributions of the corresponding P and S atoms, and the bi-atomic CN−, used for the study of the distribution of the N atoms. The three chemical images N, S and P have been obtained simultaneously, from a surface typically 40 × 40 μm. The time required to obtain these images depending on many factors (intensity of the PI beam, local concentration and characteristics of the selected atoms…), may vary from several minutes to one hour. All the images have been obtained using cesium as PI, which allows the best sensitivity for the study of atoms or groups of atoms preferentially emitted as negative SI, such as P–, N− and CN−.
3 Results
3.1 Ultrastructural changes in the podocytes
Although qualified as “minimal changes”, when observed by LM, the glomerular lesions of cases 1, 2 and 3 should be qualified as severe cellular lesions when observed by TEM. These constant alterations concern both the architecture, and the intracellular structure of the podocytes.
The architectural alterations, described with precision more than 50 years ago [11], consist of: (1) a loss of the characteristic differentiation of epithelial cytoplasm into numerous foot processes which normally insert on the outer aspect of the basement membrane; and 2) the formation of a number of microvilli at the apical cell membrane (Fig. 1). These alterations result in a decrease (case 2) or a quasi-complete (cases 1 and 3) loss of interruptions or pores separating the characteristic foot processes (Figs. 1 and 2).
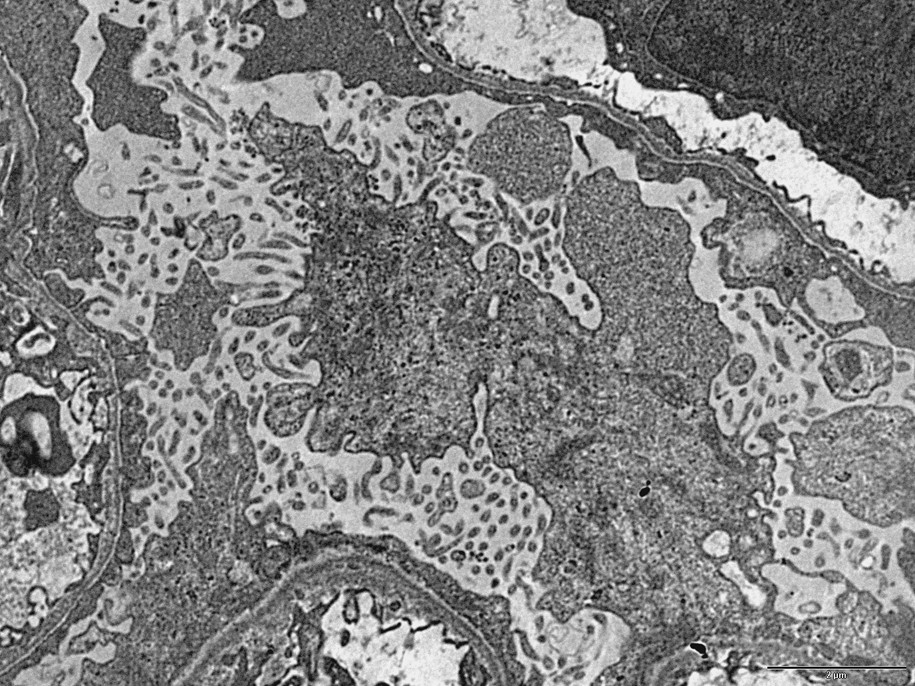
TEM image (case 1): Two architectural changes in the podocyte of a nephrotic syndrome: 1) loss of the foot processes, and 2) formation of a number of microvilli at the apical surface of the podocytes. Bar: 5μ.

TEM image (case 1): Large vacuoles in the cytoplasm of a podocyte. Bar: 5μ.
Although noted in some previous studies, lesser attention has been drawn to the intracellular alterations. These alterations concern both the cytoplasm and the nuclei.
In the cytoplasm of the podocytes (cases 1, 2 and 3), is a considerable increase in the number of ribosomes asociated with the rough endoplasmic reticulum (Fig. 3) and a dilatation of ergastoplasmic cisternae. In many podocytes, the ergastoplasm appears to be particularly developed. Besides, large vacuoles, often containing a flocculent material similar to the intracapillary plasma, are frequently observed (Figs. 2 and 5). These large vacuoles occasionally contain round electron dense bodies, 1–2 μm in diameter (Fig. 5). From one cell to another, is a variation of the intensity of these alterations: In some cells, the development of ergastoplasm being predominant, although, in other ones, the main changes consist in the presence of a number of the very large vacuoles.
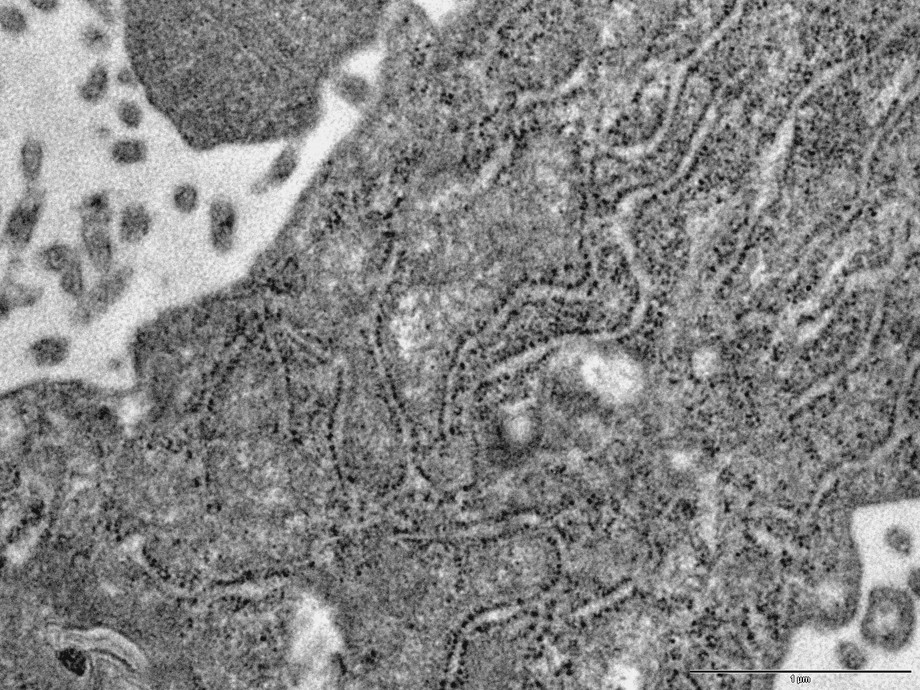
TEM image (case 1): development of the rough endoplasmic reticulum (ergastoplasm) in a podocyte. Bar: 0.1μ.
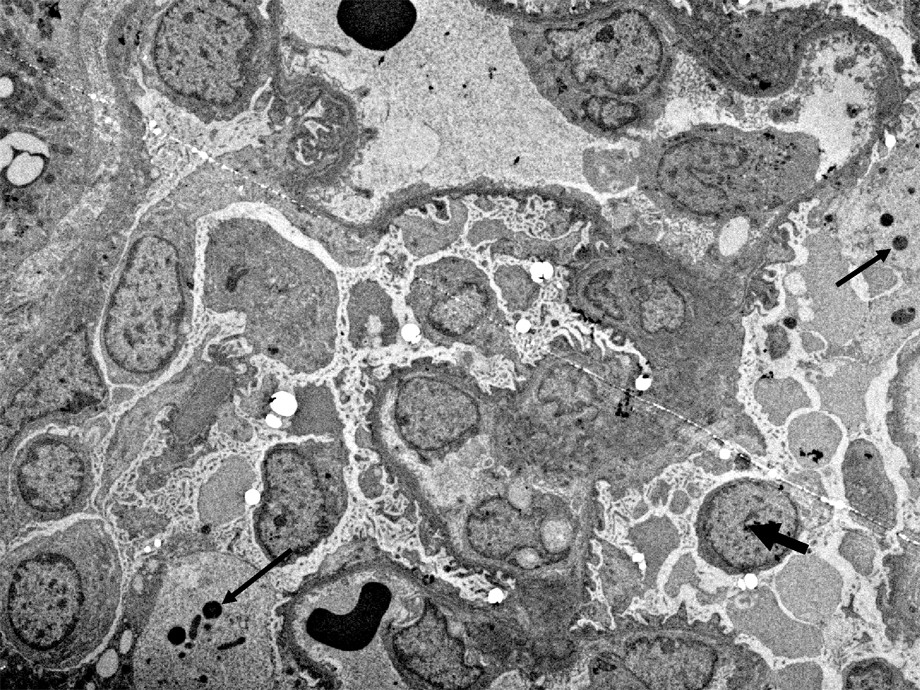
TEM image (case 2). Large vacuoles containing: a) a flocculent material, similar to the intravascular plasma and, b) dense round bodies (thin arrows). Besides, it should be noted that, in this patient, there is not a complete loss of the foot processes differentiation, and that a condensed heterochromatin is clearly visible in many nuclei, with some exceptions (Large arrow). Bar: 5μ.
In many nuclei, there are striking changes in the distribution of the chromatin, which appears homogeneous (euchromatin) all over the nucleoplasm (Fig. 4), without any condensation at the periphery (heterochromatin), usually observed in the nuclei of non-proteinuric patients. This particular change has been frequently observed in the case 1 (Fig. 4), and less frequently in the cases 2 and 3.

TEM image (case 1): Unusual change in the distribution of the chromatin, which appears homogeneous (euchromatin), in the nucleus of this podocyte.The condensation of chromatin (heterochromatin) is no longer visible. Bar: 0.1μ.
3.2 Ion microscopic findings
Striking changes in the distribution of the three elements N, S, and P are observed in both the cytoplasm and the nuclei of the podocytes of proteinuric patients.
In the cytoplasm, the large vacuoles frequently contain small round bodies (case 2), about one μm in diameter, with a higher concentration of N and S (without P) suggesting a high concentration of proteins. These round bodies probably correspond to the dark round bodies observed by TEM (Fig. 5). Similar round bodies containing a high amount of N and S are also observed in the cytoplasm of a number of Proximal Tubule Cells (PTC) of the three studied NS whereas, in PTC, the number of these bodies is higher in case 1 (Fig. 8).
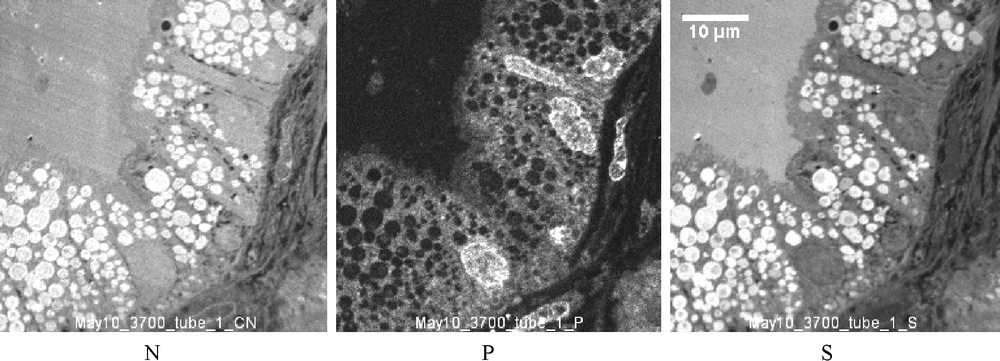
SIM images (case 1): Distribution of N, P and S in PTC. In the cytoplasm are a number of round bodies containing N and S at a high concentration and which should be compared with the intravacuolar round bodies described in Fig. 6. However, this present work has been focused on the podocytes, and only a few PTC have been studied by TEM and SIM. This number is insufficient for a realistic evaluation of a possible protein synthesis inside the tubular cells.
In the nuclei, the microscopic distributions of the three elements N, S and P vary greatly from one nucleus to another, as shown in Fig. 6. These differences, never observed in non-proteinuric patients, should be compared to the changes in the distribution of euchromatin and heterochromatin revealed in the TEM images.
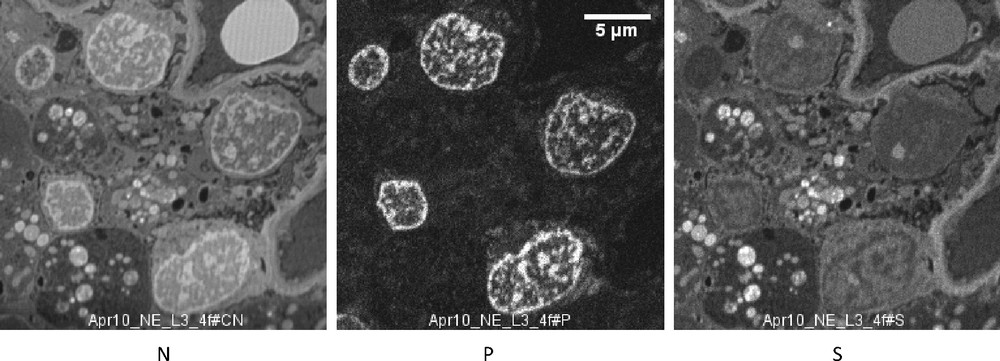
SIM images (case 2). N, P and S distribution in five podocytes. In the cytoplasm are large vacuoles containing a number of round bodies containing a high concentration of N and S, without P. These chemical images should be compared with the electron microscopic image taken in the same patient (Fig. 5), where the intravacuolar dark bodies probably correspond to the round dense bodies rich in N and S. Bar: 5μ.
4 Discussions
An excess of protein in the final urine may have different origins such as: (1) a defect of the GFB perm selectivity which allows the proteins to cross the barrier, and to be released into the final urine after a possible modification along the nephron; (2) A tubular secretion of protein from the blood; or (3) an excess of protein synthesis inside the epithelial cells (glomerular or tubular), followed by a release of the secreted proteins into the urinary space.
4.1 Previous TEM studies and mechanism of human proteinuria
The possible role of a defect in the perm selectivity of one of the three constitutive layers of the GFB revealed by TEM, successively the fenestrated endothelium, the glomerular basement membrane (GBM) and the epithelium layer where the foot processes are inter-connected by the slit diaphragm, has been debated, and, for a long time, the barrier was considered to be a size, shape or charge-selective filter. In the 1950s, a selective filter of macromolecules seemed to be displayed in the epithelial layer of the GFB, with the description by TEM [12] of the organization into pedicels of the cytoplasm of podocytes at the outer side of the GBM, the small spaces (3–4 nm), separating epithelial foot processes being of the order of the diameter of albumin. Less than six years later, this simple explanation had to be definitely shelved with the description by M. Farquhar et al. [11], demonstrating without ambiguity that, in NS, the epithelial cytoplasm coverts almost the entire surface of the GBM with a quasi-complete loss of interruptions or pores. These findings, being largely confirmed in all the following studies, the epithelial layer of the GFB could no more be considered as a passive filter, which led to support the Rinehart's concept of a glomerular filtration [13] where the filtrate pass through a living cell: the podocyte. In the same way, the concept of a structural basis of proteinuria based on a defect of the glomerular basement membrane has been discussed with the description in the earliest TEM studies of some ultrastructural alterations of the GBM in the NS. Again, this concept had to be rapidly shelved with the following works demonstrating that: (1) in most NS, no evidence of any ultrastructural GBM alteration could be provided; and (2) in the cases where indisputable alterations are visualized such as diabetic nephropathies, electron dense alterations of the GBM [14], there is no relation between the intensity of a GBM alterations and the proteinuria.
In more recent studies, the functional importance of two additional layers, the endothelial cell layer (glycocalyx) composed of proteoglycans, glycosaminoglycans and plasma proteins and the sub podocyte space [4] has been proposed.
News insights into the biology of the podocyte have been opened with the identification of nephrin, the first slit-diaphragm protein to be identified [15], and the demonstration of a mutation of the nephrin gene in the NS of the Finish type. This discovery, followed by the identification of a number of other proteins (podocine, FAT, NEPH1, CD2- associated protein, P-cathepsin…), which are now considered as vital for the integrity of the GFB led to new concepts on the mechanism of proteinuria, and emphasizes again the importance of the slit diaphragm as a cause of the molecular sieving of the GFB, at least in some varieties of NS.
However, the precise interactions of these proteins, that are crucial for the maintenance of the GFB integrity, remain to be identified and, according to most authors, the underlying mechanism of human proteinuria remains unknown.
4.2 Contribution of the present TEM study
The current tendency to focus on the role of the podocytes already was in concordance with most previous ultrastructural studies carried out these last five decades on the biopsies of NS, where, with the exceptions of some rare diseases (dysproteinemia, tubular nephropathies), the only constant ultrastructural changes related to human proteinuria was observed in the podocytes, whatever the origin or the variety of the renal disease. Furthermore, the number of affected cells as well as the severity of the cellular changes were clearly dependent on the intensity of the proteinuria, whereas no ultrastructural evidence could be provided, supporting the role of the GFB. However, in these early TEM studies, and with only rare exceptions [11,16], attention was more focused on the architectural changes than on the intracellular alterations.
In this study, more attention has been drawn to the intracytoplasmic and the intranuclear changes. Usually observed in the cytoplasm of a normal podocyte, an octopus-shaped cell, are a variety of microstructures: a few mitochondria, lysosomes, free lysosomes, a number of microtubules, filaments (α-Actin, myosine), a large Golgi apparatus, abundant smooth endoplasmic reticulum, although the rough endoplasmic reticulum, though noticeable, remains usually weakly developed. The difference in proteinuric patients is a considerable development of the rough endoplasmic reticulum, a structure where the membrane of the reticulum is associated with a number of ribosomes forming the ergastoplasmic cisternae. This structure is well known to be associated with a specific function, a protein synthesis followed by an excretion outside of the cell of the new-formed protein. These simple ultrastructural findings give evidence of a cell synthesizing and excreting large amounts of proteins, and may explain an excess of proteins in the primitive and in the final urine.
In human disease, a direct quantitative measurement of the amount of the protein secreted from the podocytes into the primitive urine and released in the final urine, after the possible modifications along the nephron is not possible. Only indirect comparative evaluations may be proposed. For example, compared to other varieties of cells, specialized in active function of synthesis and excretion of proteins, the podocytes of patients with heavy proteinuria contain a number of ergastoplasmic ribosomes which may approach the number observed in plasmocytes or pancreatic exocrine cells (Fig. 3). Otherwise, compared to the podocytes of non proteinuric patients, the number of ergastoplasmic ribosomes may be estimated to be several orders of magnitude less. Although imprecise, these comparative evaluations suggest that, in NS, the excess of protein released from the podocytes must be considered as significant, even if a precise quantitative evaluation was not possible.
4.3 Limitations of the SIM studies
There are three main factors of limitation in the contribution of SIM in this study: (1) The tissues being chemically fixed and embedded in epon, most of the diffusible atoms or molecules have been eliminated from the specimen and, only non soluble atoms or molecules, mostly macromolecules, are representative of the in vivo microscopic distribution; (2) Under the primary ions bombardment, almost all the chemical bounds are broken; and (3) the volume pulverized under a single impact of the PI (considered as a small cylinder, 50–100 nm in diameter, and several nm in depth), gives the limit of resolution in the analytical image. Even if this volume is extremely small, the SI emitted from the pulverized crater may be issued from small fragments of several different molecules (Figs. 7 and 8).
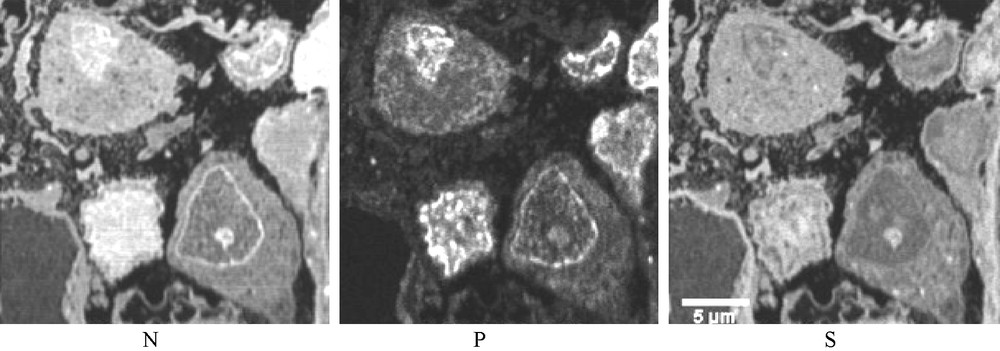
Three SIM images (case 1). N, P and S distribution in three podocytes. In the nuclei the distributions of the three elements vary greatly from one nucleus to another, probably corresponding to different phases of an unusual intranuclear activity. Such differences are not observed in the biopsies of non-proteinuric patients.
In spite of these limitations, the SIM analytical images permit the confirmation of the protein nature of the round dense bodies observed in the cytoplasm of both the podocytes and PTC of patients with heavy proteinuria (high concentration of N and S atoms without P). On the other hand, from a nucleus, where there are very complex interactions between macromolecules, it is not possible to differentiate between a protein, containing only N and S, from a chain of DNA containing only P and N or of a condensed chromatin, associated with histones, where P and N should be emitted with S. Nevertheless, in this study, the contribution of SIM, displaying very unusual distributions of these three atoms in the nuclei of podocytes permits to confirm the serious disorder in the molecular activity in podocytes of patients with heavy proteinuria.
5 Conclusion
The considerable development of ergastoplasm in the podocytes of patients with heavy proteinuria gives evidence of an excess of protein synthesis and excretion of proteins into the tubular fluid. The amount of the synthesized proteins must be considered as significant, but a precise quantitative evaluation was not possible. Beside, the nature of the proteins remains to be identified.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
This work was supported by the Conselho Nacional de Dsenvolvimento Cientifico e Tecnologico, Brasilia, Brazil. We wish to thank Marie Claire Gubler for providing the kidney tissues samples, Jean Luc Guerquin-Kern and Ting-DU Wu for providing access to the SIMS facilities of INSERM, U91, Institute Curie, University Paris X12, Orsay, France, and Claudio Figuera for providing access to the electron microscope Dept. of FIOCRUZ, Salvador, BA, Brazil.


