1 Introduction
In bird species that exhibit biparental care (about 90% of bird species: [1,2]), an evolutionary conflict arises between mates over investment in their offspring [3–5]. Each parent has limited resources to divide among reproduction and self-maintenance [6] and this generally results in a trade-off between current and future reproduction [7]. Each parent therefore benefits from effort invested by its partner and has a potential interest to reduce its own investment by leaving its partner to compensate [5,8]. How parents solve the sexual conflict over care of young has been the subject of much attention, both theoretical and empirical (reviews in [5,9]) but it is still misunderstood despite of numerous experimental studies across bird species [10]. Sexual conflict can lead to parentally biased favouritism towards particular individual offspring. Differential food allocation among sibling may result from nestling competition [11,12] or active parental decisions [13–18] or both [19]. The parental decision can be influenced by the behaviours of nestlings when parents rely on the begging intensity to favour the nestling with the highest needs (review in [10]). Offspring may manipulate parental decisions to get a greater share of care to their own advantage [10]. For instance, in the Collared Flycatcher Ficedula albicollis, food allocation is determined both by the begging intensity and the position of chicks that directly results from nestling competition [19]. The relative importance of parental and nestling control may change, depending on food abundance. When food is scarce, parents may prefer nestlings with better survival prospects [14,20–22]. In several studies, the preferential feeding of larger young has been found [23–25]. In addition, the benefits for parents to preferentially feed one type of nestlings may differ according to their sex (e.g. brood division: [26]) thereby compounding the complexity of the question. Male and female parents can indeed respond differently to the nestling begging, relative age, size and sex [15,27] leading to different long-term feeding preferences. Males generally are hypothesized to invest less time and energy in parental care and therefore to feed only the nestlings that provide the highest fitness return at the lowest possible cost [28]. However, in many shearwaters, males provide 40 to 50% more food for nestlings than do females [29]. Fathers generally tend to preferentially feed larger offspring, whereas females tend to favour small offspring [6]. Yet Tanner et al. [30] showed in Great Tits Parus major that both parents had a similar preference to feed the hungriest nestlings which begged more intensively. Inversely in budgerigars Melopsittacus undulatus, males are more responsive to begging intensity than females which seemed to assess other phenotypic characteristics of the nestlings, using size in particular to modulate their response to begging [31]. Under manipulation of partner contributions [32,33], females seem to be better informed regarding brood needs than males and are better able to adjust their effort levels to compensate changes in partner efforts. In some bird species, male and female parents may exhibit different preferences in relation to offspring sex influencing thereby the secondary sex ratio. Female parents usually provide a higher proportion of the feeds to sons than to daughters [34,35], especially when females mate with males which exhibit large secondary sexual characters (Barn Swallows Hirundo rustica: [25]). This sex-oriented preference may also be modulated by external constraints. Hence, when Zebra Finches Taeniopygia guttata parents face low food availability, mothers shift allocation to cheaper offspring, namely to sons, and fathers are able to compensate for their mates’ preferences. Under favourable conditions, females favour the expensive offspring (daughters), whereas males display no preference [36]. This result is in accordance with sex allocation theory which underlines the adaptive significance of parental behaviour's plasticity.
In a proximal point of view, parental response to chick begging behaviour may result from a conflict between “background” parental preference (e.g. female parent preferring to feed her daughters under optimal conditions) and chicks’ manipulative behaviour (e.g. female parent driven to feed preferentially a hungry male chick that begs more intensely than his siblings). Depending on the balance between both forces, a parents’ decision may or may not fluctuate between feeding events. One can imagine that parental preference can be temporarily influenced by discreet differences between chicks begging behaviour that could either result from the chicks’ strategies (competition or cooperation) or even emerge from stochastic events.
One stochastic event is likely to be found in situations where chicks beg together: synchronization between siblings may not be perfect all the time and the identity of the beggar that begins first, could be randomly distributed among siblings throughout begging events. The sensitivity of each parent to these subtle changes within the begging events remains an open question. It would be particularly interesting to ascertain whether these subtle changes could modify a parental preference for a given category of chick (e.g. female or male chick) and to obtain clues regarding the strength of the parental preference. The aim of this study was to investigate the aforementioned question in the Zebra Finch. This is a species which exhibits biparental care as a rule and where differences in sex allocation tactics have been reported [35], e.g. in response to food quality [37–39] and the body conditions of mates [36]. Using two-chick nests with a balanced sex ratio (one male and one female), we observed, firstly, if parents exhibited actual preferences for one nestling sex and, secondly, if this preference could be modulated by discreet changes within begging behaviour. This study was conducted on captive Zebra Finch families breeding in optimal conditions in order to eliminate the confounding effect of environmental pressure. Taking advantage of the fact that most of the begging sessions towards parents implied both chicks, we first investigated a possible parental preference when nestlings simultaneously initiated begging calls. In order to determine this, we examined the sex allocation of both parents in food provisioning. Then, we assessed if this parental preference could be modified by a slight time-lag between nestlings in the initiation of begging bouts.
2 Materials and methods
2.1 Subjects
Birds used in this experiment are from our laboratory stock. Females were paired with arbitrarily selected males. To study the parental food allocation before fledging, six breeding pairs of Zebra Finches with two-nestling broods (one male + one female) were video-monitored. Parents were in excellent body condition and had bred offspring prior to the present study (one clutch, within the 3 months before the experiment). To get two-nestling broods, we monitor each day breeding birds in our colony and let the two first eggs laid by females in the nests, removing the following ones (zero to two eggs removed, depending on the female). Adults were thus the biological parents of the two siblings. The age difference between both chicks within a given nest never exceeded 1 day. The number of nests where the female chick hatched before the male chick was the same as the number of nests where the male chick was the first to hatch (three nests of each). Each breeding pair was settled in separate cages (40 × 40 × 25 cm) equipped with an enclosed wicker nest with one main opening. Composite food for tropical finches and water were provided ad libitum and the room temperature was maintained at between 25 and 28 °C (14 L/10 D photoperiod). Chicks were marked with a non-toxic coloured mark on their feathers for individual identification on the video recordings (yellow mark by picric acid for one chick, methylen blue for the other one). The number of birds marked with yellow and blue marking was balanced between both sexes among nests. Molecular sexing of nestlings was performed from feathers (DNA sexing performed by GENINDEX, La Rochelle, France). Taking advantage of the adult Zebra Finch sexual dimorphism, the result of molecular sexing was further checked visually when the juveniles reached adult age.
2.2 Video and audio recordings
In order to acclimatize birds and reduce the disturbance, the video equipment was set up in the cages at least 4 days prior to the potential hatching. An IP video camera LAN (D-Link DSC-900) was focused on each nest opening. Videos were recorded at five frames/s on a Bi Xeon IntelWorkstation, which was located in a different room. IPView Lite Software version 3.88 was used and read with Media Player Classic. The six broods were video-monitored from the age of 10 days to 14 days after hatching. Before 10 days old, the chicks were often masked by parents during the feeding behaviour which impaired the identification of individuals. Some fledglings left the nest at the age of 15 days. The recordings occur throughout the day, from light onset to light offset (14 hours of recording/day). This longitudinal approach allows a comprehensive analysis of all begging events that occurred in the nests, over a period of 5 days.
The roof of nests was equipped with an omnidirectional tie microphone (AKG C417, frequency response almost flat between 50 Hz and 10 kHz). The microphone position and recording settings were standardized across all the nests. Microphones were connected via a shielded cable to the audio card of the Bi Xeon IntelWorkstation.
2.3 Behavioural analysis
Parental visits to nest induced a begging sequence from nestlings in 75% of the cases. Both nestlings used to beg together throughout the begging sequence (97% of observed begging events). A begging sequence started with the entrance of the parent into the nest and stopped with the parents’ departure. It lasted around 1 min (mean ± SD = 70 ± 45 s). The onset of a begging sequence was however not always initiated by both chicks together. It was thus possible to distinguish between begging initiated by one chick and begging simultaneously initiated by both chicks. For each begging event, the following observations/measurements were thus extracted from the video:
- • nature of the begging event: “synchronized” or “not synchronized”. The begging event was arbitrarily classified as “synchronized” when the delay between the first begging call of both chicks did not exceed 0.5 s, representing 0.7% of the total mean duration of begging bouts. The maximum time-lag between the two chicks never exceeded 2 s;
- • identity (and thus sex) of the first begging nestling;
- • sex of the feeding parent;
- • number of parental regurgitations for each chick.
From these measurements, we calculated for each day and each parent, the proportion of “synchronized” and “not synchronized” begging events. To assess the consistency of a possible parental preference towards one chick sex, we then separately calculated for each type of begging event the proportion of days when the parent favoured one of the nestlings. When the difference in the number of regurgitations received between the two nestlings exceeded 5%, we considered that nestling to be favoured. As chicks were fed several hundreds times a day (see results), the 5% threshold represents a difference between both chicks of more than 10 parental regurgitations. Lastly, we measured the possible change in food allocation of each parent by comparing the parental preference exhibited when the begging event was synchronized and not synchronized for each nest. For each parent in each nest, we calculated the difference in the proportion of days when the female chick was preferred between synchronized and not synchronized begging events. When the difference was null, it meant that the parental preference was maintained whatever the type of begging event. A null difference may thus be observed when a female chick was favoured in 100% of monitored days in both synchronized and not synchronized begging events (Table 1). When the difference was positive, it meant that the parental preference was reinforced. Inversely when it was negative, the preference was reduced.
Comparison of parental preference for female nestlings when begging events were synchronized and not synchronized.
| Female parents | Male parents | |||||||||
| % of days with a preference for F nestling when begging events were: | ≠ of preference for F nestling between synchronized and not synchronized begging events | % of days with a preference for F nestling when begging events were: | ≠ of preference for F nestling between synchronized and not synchronized begging events | |||||||
| synchronized | not synchronized | synchronized | not synchronized | |||||||
| Nest | M | F | M | F | M | F | M | F | ||
| 1 | 100 | 75 | 60 | –25 | –40 | 100 | 0 | 100 | -100 | 0 |
| 2 | 100 | 0 | 100 | –100 | 0 | 75 | 25 | 100 | -50 | 25 |
| 3 | 100 | 60 | 100 | –40 | 0 | 40 | 20 | 80 | -20 | 40 |
| 4 | 40 | 0 | 100 | –40 | 60 | 20 | 0 | 100 | -20 | 80 |
| 5 | 20 | 20 | 80 | 0 | 60 | 20 | 0 | 20 | -20 | 0 |
| 6 | 40 | 20 | 80 | –20 | 40 | 100 | 0 | 100 | -100 | 0 |
3 Results
3.1 Nestlings’ begging behaviour
We recorded an average of 107 ± 40 begging events/day per nest, equally addressed to female and male parents (51 ± 15 and 57 ± 29 respectively, Wilcoxon matched pairs test n = 30, z = 0.715, P = 0.47; six nests monitored 5 consecutive days). Overall, 61% of the begging events were “not synchronized” (n = 3373 total begging sequences involving both nestlings). The sex of parents influenced the occurrence of the “not synchronized” begging events. The mean proportions of “not synchronized” begging events among the six nests for each day were significantly higher when male parents visited nest than when female parents visited nest (Sign test for small sample, n = 6, P = 0.04). On average, 56% of begging events (50–68%) addressed to fathers were “not synchronized” and only 30% to female parents (28–34.5%). This observation was consistent whatever the age of nestlings (Friedman Anova n = 5 days, df = 4: male parents χ2 = 1.43, P = 0.9; female parents, χ2 = 7.16, P = 0.2). Female nestlings initially begged more often than males (55 ± 18% of “not synchronized” begging bouts/day initiated by females, Wilcoxon matched pairs test n = 30, z = 2.48, P = 0.013). This difference was consistent whatever the age of nestlings (Friedman Anova n = 5, df = 5: χ2 = 1.14, P = 0.95).
3.2 Parental responses
We focused on parental visits which induced begging behaviours (75% of their visits). The parental investment of males and females were similar (female parent: 240 ± 85 regurgitations/day; male parent: 233 ± 107 regurgitations/day, Wilcoxon matched pairs test n = 30, z = 0.4, P = 0.69). When nestlings beg synchronously, mothers exhibited a consistent preference throughout the time period for the female nestling in three nests out of six (nests 1, 2 and 3; Fig. 1a), meaning that the female parents gave more regurgitation to the female chick than to the male. In the three other nests, no stable favouritism was observed for a specific sex. We could therefore hypothesize that the parental preference in ‘synchronized’ begging events might be influenced by the fact that female nestlings beg first more often than males (see Results above). However, female nestlings begged first in a similar proportion in nests 1, 2, 3 and 4, 5, 6 (Mann Whitney z = 0.516, P = 0.62; nests 1, 2 and 3: female nestlings begged first in 54% of “not synchronized” begging events [median]; nest 4, 5 and 6: median = 57%). Fathers exhibited a consistent preference for female nestling in two nests (nests 1 and 6, Fig. 1b). A similar tendency was recorded in nest 2 where the father gave more regurgitation to the female nestling during 3 consecutive days. Inversely, one male nestling was preferred by the male parent in nest 5 during 4 consecutive days out of 5 of monitoring. Lastly, no consistent preference from fathers was observed in two nests (nests 3 and 4, Fig. 1b) throughout the development of nestlings.
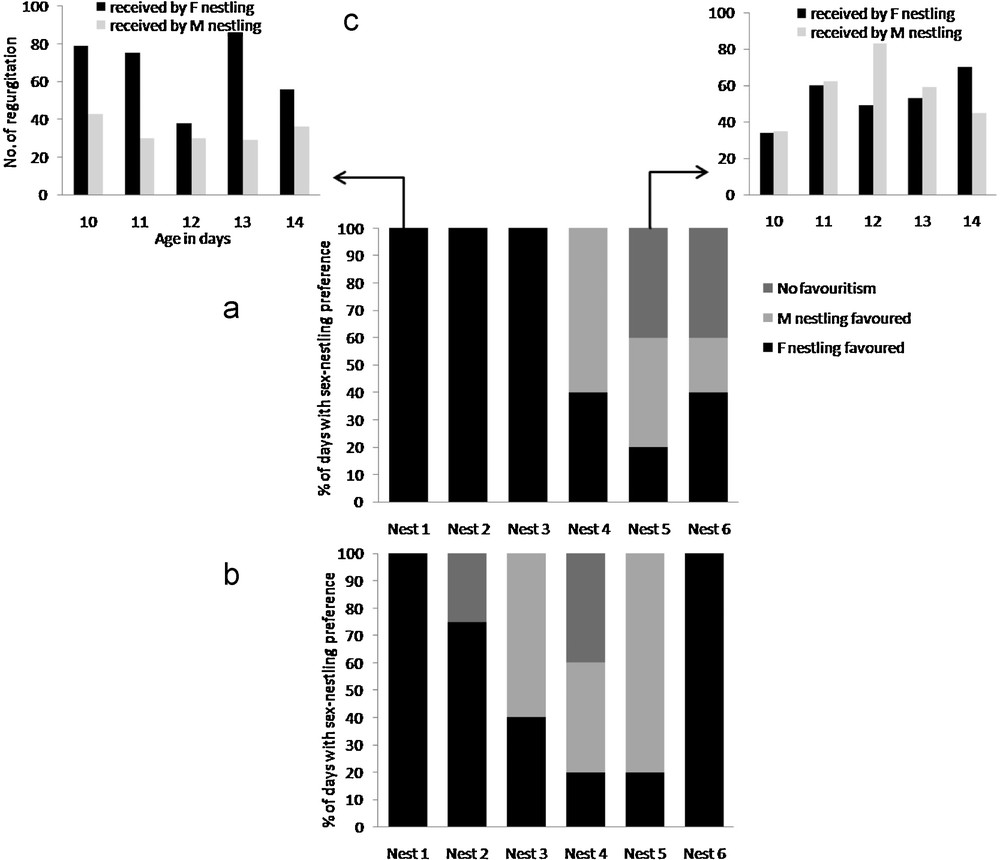
Percentage of days when parents exhibited a preference for one-sex nestling (giving more regurgitation) when nestlings begged synchronously; 1a: female parents; 1b: male parents; 1c: number of daily regurgitation given by female parent in nest 1 and 5; M: male; F: female.
Parental sex preference in food provisioning could be modulated by a discreet asynchrony between the first begging call of both nestlings, with a benefit for the first begging individual (Table 1). When male chick begged first, mothers thus diminished their female-directed feeding preference. The difference between preference for the female chick during “synchronous” begging and preference for the female chick during begging initiated by the male chick was however not significant (Sign test for small sample: n = 6, P = 0.07). When a female chick begged first, the female preference for the female chick was reinforced in the three nests where this preference was not prominent during “synchronized” begging, and was maintained in two other nests (Table 1). Male parents systematically fed preferentially the nestling begging first (Sign test for small sample: significant difference between preference for female chick during synchronous begging and preference for female chick during begging initiated by male chick, n = 6, P = 0.041). Preference of male parents for the female chick was reduced when the male chick begged first and this was reinforced when female chick begged first. Although both male and female parents were sensitive to this “first come, first served” rule, the amplitude of this modulation tended to differ between male and female parents. Male parents appeared to respond uppermost to the nestling that begged first (three nests out of six, Fig. 2b). Although female parents could also favour the first nestling begging (two nests; Fig. 2a), their preference for their daughter was confirmed in two of the nests where their sons begged first (nests 1 and 3).
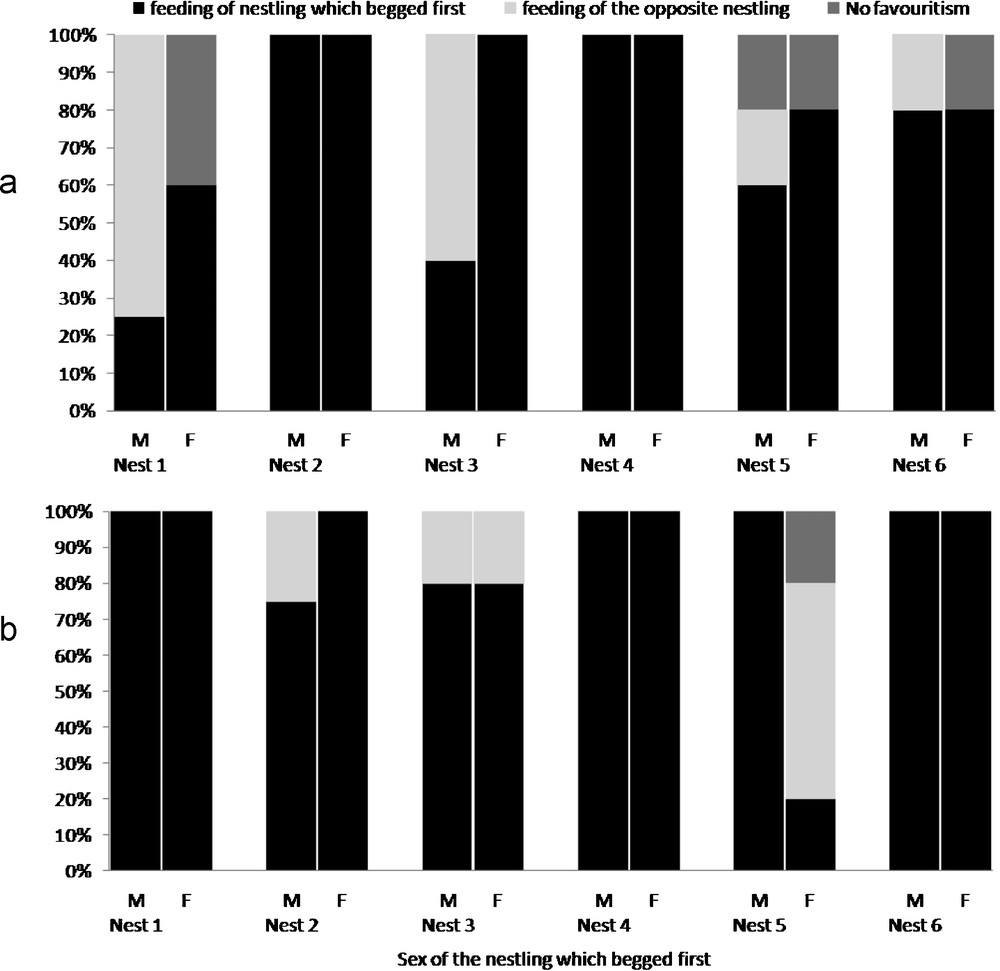
Percentage of days when parents favoured one nestling sex (giving more regurgitation to it) when nestlings begged with a time-lag; 2a: female parents; 2b: male parents.
4 Discussion
Although we have to remain cautious due to the sample size, our results support the hypothesis that the fine dynamics of chicks’ begging behaviour could modulate parental preferences. Different patterns of food allocation were observed among nests and between parents within a nest. Under optimal conditions, Zebra Finch parents consistently favoured one offspring sex in half of studied nests. We observed however that the begging behaviours of offspring influenced the parental response. A discreet change, such as a short delay in the initiation of begging calls between nestlings, could therefore modulate the parental preference. The female parent appeared less sensitive to this chick-induced modulation than the male parent. Interestingly, the occurrence of not synchronized begging events was more frequent when the most tractable parent (male) visited nest. This parental preference means that parents can reliably discriminate between their chicks. This discrimination could rely on the individual identity of nestlings since a vocal signature was established early within Zebra Finches [40]. This preference was however mostly expressed in favour of female nestlings, and parents may also be able to discriminate the sex of their offspring.
Our observations showed that both parents tented to feed preferentially the nestling that begged first. We ignored that this nestling may be the hungriest one or if this occurred by chance. We did find that some female parents biased allocation toward females, the sex with higher variance in reproductive success as to be expected when resources levels are high [39,41]. They benefit more by producing daughters rather than sons because females show reduced fecundity as adults if they fledge at low body mass.
This study was preliminary since many complementary factors may influence the parental decisions such as the relative age or size of offspring. A point is that the prior breeding experience of parents could influence their behaviour: maybe parents could alter their responsiveness to chick begging on the basis of their previous success — or no success — in breeding. Further experimental studies are also needed to assess the potential changes in parental investment after food deprivation and to test the importance of parental decisions in food provisioning. In conjunction with this, it would be interesting to perform — under controlled environmental conditions — playback experiments using modified signals from female and male nestlings in order to test the ability of sex recognition of parents. In addition to possible sex difference in physical structure of calls, sex-specific visual displays during begging behaviour may exist in Zebra Finch as it has been shown in barn swallows. In Barn Swallows, a sex difference in the mouth coloration was first observed which later disappeared, and a sexual vocal signature took place as a second stage [42]. Furthermore, a study of the House Sparrow Passer domesticus revealed that parents use visual cues such as mouth colours to bias resource allocation towards the healthiest rather than the most needy offspring [18]. Other sex-specific characters may signal the sex to parents and would need to be investigated in Zebra Finch.
To conclude, offspring might benefit to be sexually identifiable to influence the parental response. These results raise the evolutionary question of why the disfavoured sex does not conceal its sex by mimicking the other sex's characteristics. Nestlings could recognize the sex of their broodmates, e.g. through begging vocalizations, and adjust their competitive behaviour accordingly. The outcome of parent-offspring conflict is under the control of permanent feedbacks between parental decisions and the manipulative behaviours of nestlings. The offspring's development strategy and the parent's investment strategy are entwined within an evolutionary game which makes the study of the communication network fascinating.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
This work was supported by the French National Research Agency (ANR, project “BIRDS’VOICES”). NM is funded by the Institut universitaire de France (IUF). We are grateful to Colette Bouchut and Nicolas Ogier for technical assistance, and Mark van Niekerk for revising the English of the manuscript.


