1 Introduction
During the last years, several studies have been carried out concerning the ultrastructural characters of spermiogenesis and/or the spermatozoon in digenean trematodes [1–11]. All these studies have mentioned the usefulness of ultrastructural spermatozoa characters in understanding systematic and phylogenetic relationships within the Digenea.
The present contribution represents the first study concerning the description of the ultrastructure of both spermiogenesis and mature spermatozoon of the gyliauchenid species Robphildollfusium fractum Paggi and Orecchia, 1963 and the fifth within the superfamily Lepocreadioidea Odhner, 1905, which includes the family Gyliauchenidae Fukui, 1929. Moreover, the present article constitutes the first description of spermiogenesis in a gyliauchenid. In addition to our contribution towards increasing the ultrastructural knowledge of digenean spermatozoa, we also compare spermatological features of R. fractum to those of other digeneans, particularly lepocreadioideans, in order to highlight the phylogenetic usefulness of these ultrastructural characters.
2 Materials and methods
2.1 Specimens
Live adult specimens of R. fractum were collected from the digestive tract of a naturally infected Sarpa salpa (Teleostei, Sparidae) captured off the coast of Dakar (Senegal).
2.2 Microscopy
After dissection, live digeneans were routinely processed for TEM examination. They were fixed in cold (4 °C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.4 for a minimum of 2 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.4, postfixed in cold (4 °C) 1% osmium tetroxide in the same buffer for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.4, dehydrated in an ethanol series and propylene oxide, and finally embedded in Spurr resin. After localization of testes and seminal vesicle in semithin sections, ultrathin sections were made using a Reichert-Jung Ultracut E ultramicrotome, placed on copper grids and double-stained with uranyl acetate and lead citrate according to the Reynolds [12] technique. To evidence glycogen, gold grids were also processed according to the Thiéry [13] test. They were treated in periodic acid, thiocarbohydrazide, and silver proteinate (PA-TCH-SP) as follows: 30 min in 10% PA, rinsed in milli-Q water, 24 h in TCH, rinsed in acetic solutions and milli-Q water, 30 min in 1% SP in the dark, and rinsed in milli-Q water. All ultrathin sections were examined using a JEOL 1010 transmission electron microscope operating at 80 kv.
3 Results
3.1 Spermiogenesis
The main stages of spermiogenesis in R. fractum are illustrated in Figs. 1–9.

Spermiogenesis of Robphildollfusium fractum. (1) Longitudinal section of a differentiation zone showing the orthogonal development of one flagellum and the intercentriolar body. (2) Detail of the intercentriolar body. (3-4) Longitudinal sections in the differentiation zone when flagella begin their rotation and become parallel to the median cytoplasmic process. Note also nuclear migration in this area. (5-6) Several cross-sections in the proximal area of the differentiation zone showing nucleus in migration and in the distal area exhibiting only median cytoplasmic processes and free flagella. Note the attachment zones in the median cytoplasmic processes (arrowheads). AM: arched membranes; C: centriole; CM: cortical microtubules; F: flagellum; IB: intercentriolar body; MCP: median cytoplasmic process; N: nucleus; SR: striated rootlets. Scale bars: 0.5 μm (Figs. 1, 3, 4), 0.2 μm (Fig. 2), 0.3 μm (Figs. 5 and 6).

Spermiogenesis of Robphildollfusium fractum. (7) Longitudinal section before the fusion of flagella with the median cytoplasmic process in which striated rootlets are still present in the spermatid. (8) Advanced stages during the constriction of the ring of arched membranes showing mitochondrion in migration. AM: arched membranes; Ax: axoneme; C: centriole; M: mitochondrion; PDM: pear shaped electron-dense material; SR: striated rootlets. Scale bars: 0.5 μm.
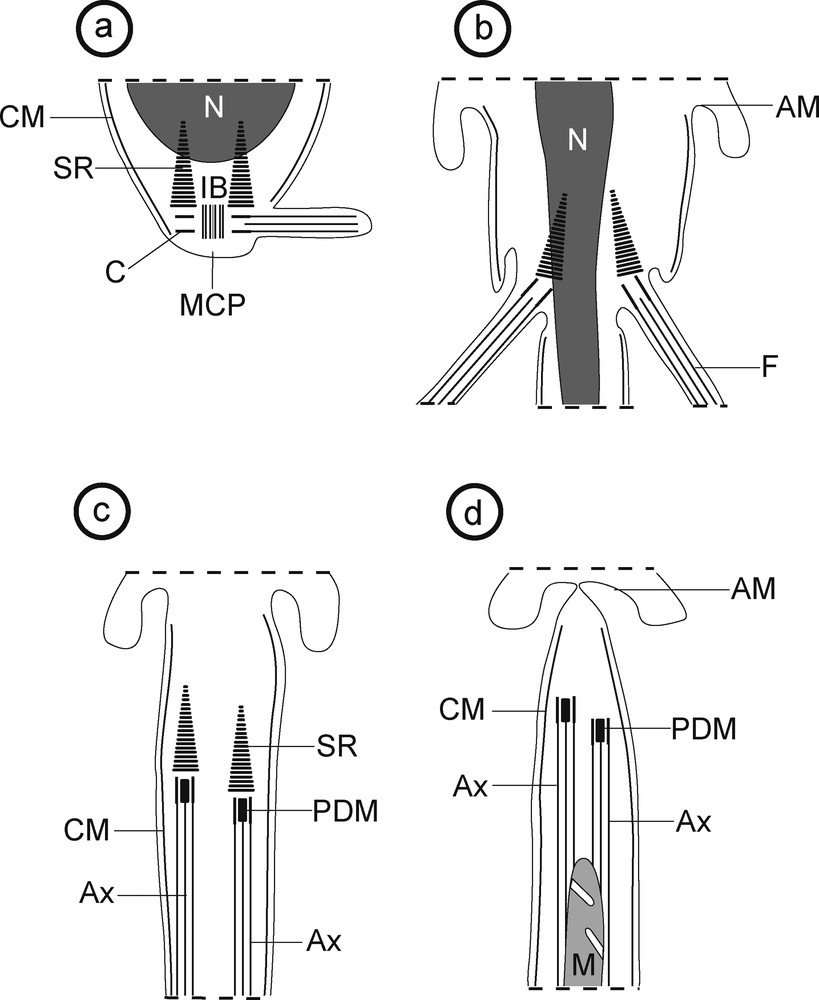
a–d: schematic drawing showing the main stages of spermiogenesis of Robphildollfusium fractum. AM: arched membranes; Ax: axoneme; C: centriole; CM: cortical microtubules; F: flagellum; IB: intercentriolar body; M: mitochondrion; MCP: median cytoplasmic process; PDM: pear shaped electron-dense material; N: nucleus; SR: striated rootlets.
Spermiogenesis begins by the formation of the differentiation zone in which each centriole, associated with a striated rootlet, originates a free flagellum that grows orthogonally to a median cytoplasmic process (Figs. 1, 3 and 9a). Within this differentiation zone, there is also an intercentriolar body with seven electron-dense layers (Fig. 2). Later, both flagella begin their rotation to become nearly parallel to the median cytoplasmic process (Figs. 4 and 9b). In this stage cross and longitudinal sections of the proximal area of the differentiation zone show the nucleus in migration into the median cytoplasmic process (Figs. 4 and 5) while sections of the distal area show only the median cytoplasmic process (Fig. 6). Moreover, in this stage four attachment zones are already present in the median cytoplasmic process (Figs. 5 and 6). After the proximodistal fusion of the flagella with the median cytoplasmic process, the ring of arched membranes initiates its constriction (Figs. 7–9c,d). In an early stage of this progressive constriction, the striated rootlets are still present in the spermatid body and a pear shaped electron-dense material is observed as a central element of both centrioles (Figs. 7 and 9c). In the final stage of the constriction of the ring of arched membranes, the striated rootlets disappear and it is noticeable that the mitochondrion is still migrating (Figs. 8 and 9d).
3.2 Spermatozoon
The interpretation of several cross and longitudinal sections of the mature spermatozoon of R. fractum allows us to establish three distinctive regions from the anterior to the posterior spermatozoon extremity (Figs. 10–30).
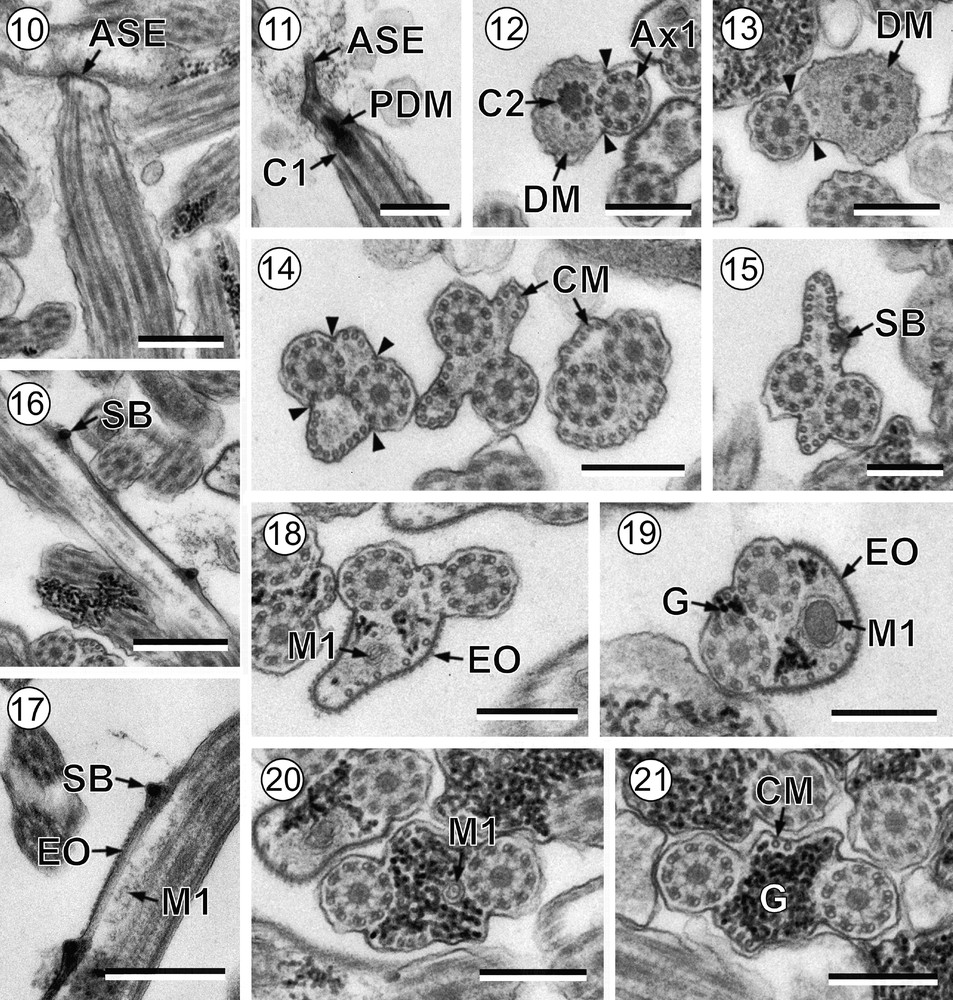
Mature spermatozoon of Robphildollfusium fractum. (10-11) Longitudinal sections in the anterior spermatozoon extremity. (12-13) Cross-sections of anterior areas of region I showing the electron-dense material and only two attachment zones. (14) Cross-sections exhibiting two parallel bundles of cortical microtubules. (15-16) Cross and longitudinal section showing spine-like bodies in an area lacking external ornamentation. (17) Longitudinal section showing the presence of spine-like bodies in the transition between non-ornamented and ornamented areas. (18–20) Cross-sections of region I showing the first mitochondrion and the progressive appearance of granules of glycogen. (21) Cross-section of region II. ASE: anterior spermatozoon extremity; Ax1: first axoneme; C1: centriole of the first axoneme; C2: centriole of the second axoneme; CM: cortical microtubules; D: doublet; DM: electron-dense material; EO: external ornamentation of the plasma membrane; G: granules of glycogen; M1: first mitochondrion; PDM: pear shaped electron-dense material; SB: spine-like body; arrowhead: attachment zones. Scale bars: 0.5 μm (Figs. 10, 16, 17); 0.3 μm (Figs. 11–15, 18–21).
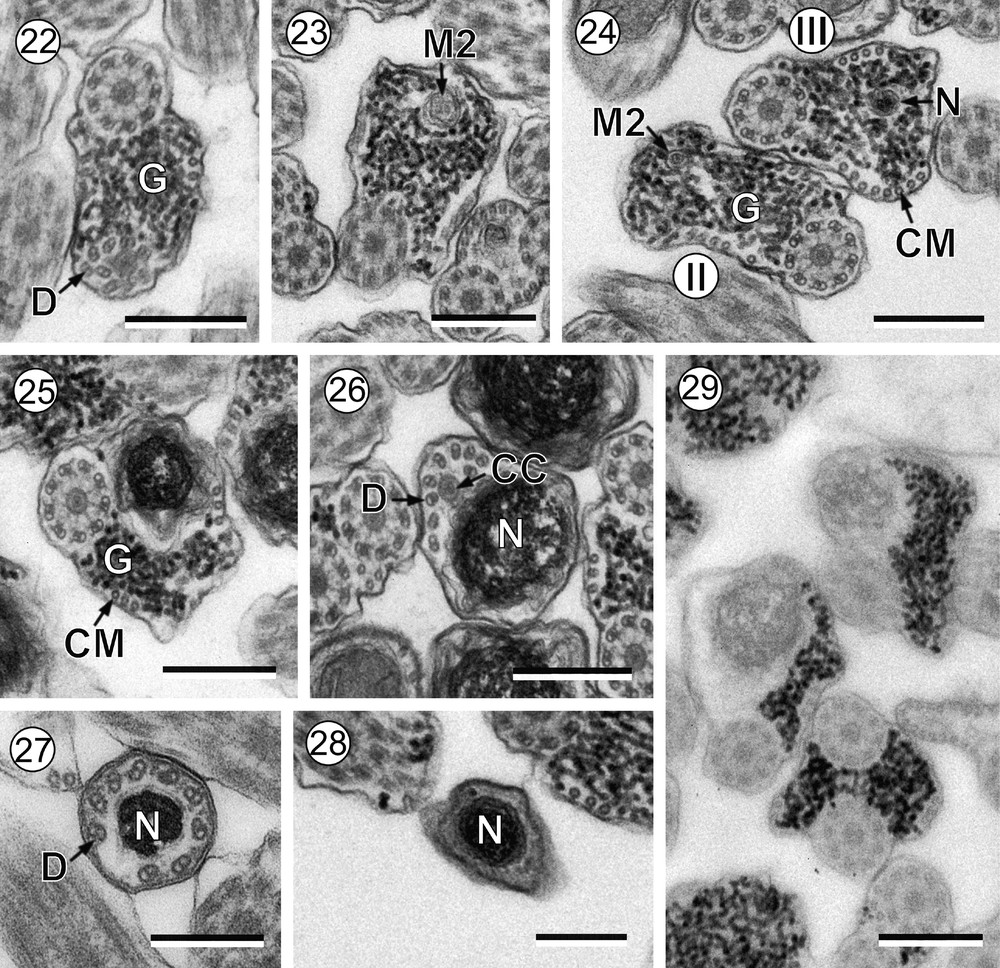
Mature spermatozoon of Robphildollfusium fractum. (22) Cross-section showing the disorganization of the first axoneme. (23) Cross-section showing the second mitochondrion. (24) Two cross-sections showing the second mitochondrion and the nucleus in the transitional area between regions II and III. (25-28) Consecutive cross-sections in the nuclear region showing reduction of cortical microtubules, disorganization of the second axoneme and posterior tip of the spermatozoon. (29) Revelation of the granules of glycogen according to the Thiéry's test. CC: central core; CM: cortical microtubules; D: doublet; G: granules of glycogen; M2: second mitochondrion; N: nucleus. Scale bars: 0.3 μm.
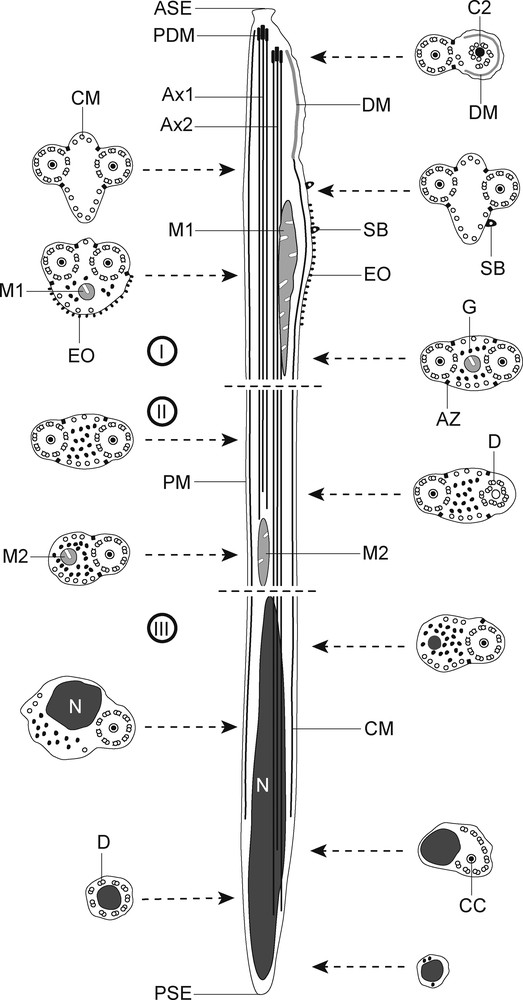
I–III: schematic drawing of the mature spermatozoon of Robphildollfusium fractum. ASE: anterior spermatozoon extremity; Ax1: first axoneme; Ax2: second axoneme; AZ: attachment zones; C2: centriole of the second axoneme; CC: central core; CM: cortical microtubules; D: doublet; DM: electron-dense material; EO: external ornamentation of the plasma membrane; M1: first mitochondrion; M2: second mitochondrion; N: nucleus; PDM: pear shaped electron-dense material; PM: plasma membrane; PSE: posterior spermatozoon extremity; SB: spine-like body.
Region I or anterior spermatozoon extremity (Figs. 10–20 and 30I) is characterized by the presence of two axonemes of the 9 + ‘1’ trepaxonematan pattern, a pear shaped electron-dense material at the level of centrioles, an electron-dense material at the periphery of spermatozoon, external ornamentations of the plasma membrane, parallel cortical microtubules, spine-like bodies and the first mitochondrion. This region could be subdivided in three parts:
- • the anterior part of region I exhibits the anterior spermatozoon extremity with a sharp tip (Figs. 10 and 11). The two axonemes are slightly displaced longitudinally to each other (Fig. 12). In the area containing the two axonemes, there is an electron-dense material beneath the plasma membrane surrounding one of the axonemes and only two cortical microtubules (Figs. 12 and 13). It is also remarkable the presence of only two attachment zones (Figs. 12 and 13);
- • in the middle part of region I the number of cortical microtubules increases and show its typical pattern into two fields, each limited by the two attachment zones (Figs. 14 and 15). This area exhibits the maximum number of cortical microtubules, six on the dorsal side and 15 on the ventral side (Figs. 14 and 15). This area also shows spine-like bodies (Figs. 15 and 16);
- • the posterior part of region I is characterized by the presence of the first mitochondrion and the reduction in the number of cortical microtubules (Figs. 17–20). External ornamentations of the plasma membrane, spine-like bodies and granules of glycogen are also present (Figs. 17–20). It is interesting to note that spine-like bodies probably mark the transition from the non-ornamented to the ornamented portion of region I (Figs. 15–17).
Region II (Figs. 21–24, 29 and 30II) corresponds to a transitional region before the nuclear area. Cross-sections in this region show the two axonemes, a reduced number of parallel cortical microtubules divided into two submembranous bundles and granules of glycogen (Figs. 21 and 22). Note the disorganization of one axoneme before the appearance of a second mitochondrion located between the disorganized area of the axoneme and the appearance of the nucleus (Figs. 22–24).
Region III (Figs. 24–29 and 30III) represents the nuclear region and the posterior spermatozoon extremity. In its proximal area, cross-sections show the nucleus, one axoneme and granules of glycogen and cortical microtubules with progressive reduction (Figs. 24 and 25). When cortical microtubules disappear, cross-sections are characterized only by the presence of nucleus and the disorganized axoneme, which exhibits the nine doublets and the central core (Fig. 26). Additionally, during the disorganization of the second axoneme, doublets are located around the nucleus (Fig. 27). Finally, the posterior spermatozoon tip exhibits only the nucleus (Fig. 28). Glycogen was evidenced by the test of Thiéry (Fig. 29).
4 Discussion
4.1 Spermiogenesis
Concerning the superfamily Lepocreadioidea, spermiogenesis has been described in two species: the apocreadiid Neoapocreadium chabaudi [14] and the deropristid Deropristis inflata [15]. Spermiogenesis in these species is similar to that observed in R. fractum in the present study and in most digenean species. It is characterized by the formation of the differentiation zone surrounded by cortical microtubules, delimited at its base by a ring of arched membranes and containing a nucleus, numerous mitochondria, and two centrioles associated with striated rootlets and with an intercentriolar body. The centrioles originate two free flagella that grow orthogonally to the median cytoplasmic process. A flagellar rotation of 90° is also reported in most digeneans [5,6,16–19], although a flagellar rotation greater than 90° is described in some species [7,20–24].
Concerning the intercentriolar body, seven electron-dense layers are observed in R. fractum and N. chabaudi [14] as described in general in Digenea [9,10]. In the case of D. inflata, the intercentriolar body is constituted of six electron-dense plates [15]. Other digeneans exhibit an intercentriolar body formed by five to seven [21] or nine electron-dense layers [7].
Both flagellar rotation and the number of electron-dense layers of the intercentriolar body have been regarded to be interesting arguments in the interpretation of digenean relationships [9,17].
4.2 Spermatozoon
Most ultrastructural characters described previously in digeneans are present in the mature spermatozoon of R. fractum. These are: two axonemes of the 9 + ‘1’ pattern of the Trepaxonemata [25], two parallel bundles of cortical microtubules, two mitochondria, a nucleus and granules of glycogen. In addition to these characters, the presence/absence of an external ornamentation of the plasma membrane, a pear shaped electron-dense material, spine-like bodies, an electron-dense material beneath the plasma membrane, and the morphologies of both anterior and posterior spermatozoon extremities are also compared, particularly with the Lepocreadioidea.
An anterior spermatozoon extremity containing two axonemes very slightly longitudinally displaced to one another has been reported in R. fractum and in all the lepocreadioid species described until now (Table 1). However, some differences were noted within the studied Lepocreadioidea. In fact, an electron-dense material appears around the second axoneme in the lepocreadiid Holorchis micracanthum [26] and in the gyliauchenid Gyliauchen sp. [27] as observed in R. fractum (present study). However, this electron-dense material was absent in the apocreadiid N. chabaudi [14] and in the deropristid D. inflata [15]. With respect to N. chabaudi [14], the authors observed cortical microtubules accompanied by external ornamentation of the plasma membrane in the tip of the spermatozoon.
Ultrastructural characters of spermiogenesis and the spermatozoon in the Lepocreadioidea.
| Family and species [reference] |
Spermiogenesis | Spermatozoon | ||||||
| IB | FR | ASE | DM | EO | SB | M | PSE | |
| Apocreadiidae | ||||||||
| Neoapocreadium chabaudi [14] | 7 | 90° | 1 Ax + CM + EO | – | + | + | 2 | N |
| Deropristidae | ||||||||
| Deropristis inflata [15] | 6 | 90° | 1 Ax | – | + | – | 2 | 1 Ax |
| Gyliauchenidae | ||||||||
| Gyliauchen sp. [27] | N/A | N/A | 1 Ax | + | + | + | 1 | 1 Ax |
| Robphildollfusium fractum [present study] | 7 | 90° | 1 Ax | + | + | + | 2 | N |
| Lepocreadiidae | ||||||||
| Holorchis micracanthum [26] | N/A | N/A | 1 Ax | + | + | – | 1 | 1 Ax |
Concerning the pear shaped electron-dense material, it has previously been observed during spermiogenesis in D. inflata, Echinostoma caproni and Brachycoelium salamandrae [15,28,29]. However, the present study constitutes the first description of this character in the mature spermatozoon.
An association of external ornamentation and spine-like bodies was also observed in the anterior area of the spermatozoon in most digeneans [4,6,10,30,31]. However, there are other digeneans presenting spermatozoa with external ornamentation but lacking spine-like bodies. This is the case of the faustulid Pronoprymna ventricosa [32], the phaneropsolid Postorchigenes gymnesicus [33], or the zoogonid Diphterostomum brusinae [34]. Moreover, in several older studies previous to the description of spine-like bodies [4], these structures could have been misinterpreted, considered as artefacts or omitted, as occurred in Haematoloechus sp. [35] and Paragonimus ohirai [36]. Within the Lepocreadioidea, spine-like bodies are absent in the mature sperm of D. inflata [15] and H. micracanthum [26] (Table 1), while in N. chabaudi [14] and Gyliauchen sp. [27], they are present but not in the ornamented area of the spermatozoon. Concerning R. fractum (present study), spine-like bodies have been observed in both areas. Thus, these structures mark the transition between ornamented and not ornamented area. The particular disposition of spine-like bodies in relation to the ornamented area needs more attention in phylogenetic analyses within the Lepocreadioidea.
Variability of the number of mitochondria is observed within the digeneans in general and in the studied lepocreadioid species in particular (Table 1). Although the determination of the number of mitochondria depends on each author's considerations [11,17,37], at least one mitochondrion is observed in all digenean species studied until now. This criterion would be of great interest in phylogenetic interpretations within the Platyhelminthes considering that the absence of mitochondrion is a recognized synapomorphy for the Eucestoda [10,27].
The posterior spermatozoon extremity of R. fractum containing the nucleus would correspond to a type 2 posterior spermatozoon extremity [38], according to the terminal character in the posterior tip. This morphology of posterior spermatozoon extremity has been described in N. chabaudi [14] and in most digenean spermatozoa [10]. In the remaining Lepocreadioidea studied until now, the posterior spermatozoon extremity contains one axoneme (Table 1). Additionally to these morphologies, a posterior spermatozoon extremity ended by cortical microtubules has been reported in other digeneans, particularly those belonging to the family Opecoelidae [4,5,8,23,30]. Moreover, a posterior spermatozoon extremity containing a mitochondrion has been described recently in the lecithasterid Aponurus laguncula [38]. All these morphologies concerning the posterior spermatozoon extremity emphasise the usefulness of this criterion when establishing spermatozoon models within the Digenea.
The spermatozoon of R. fractum possesses a short axoneme that does not reach the nuclear region. Indeed, several cross-sections in the nuclear region show only an axoneme accompanied by the nucleus and cortical microtubules. This fact could distinguish the mature spermatozoon of R. fractum from those of the remaining lepocreadioids and in particular from the sperm cell of Gyliauchen sp., which belongs to the same family [27].
Comparing molecular and ultrastructural results, some congruencies could be supported. Indeed, the Gyliauchenidae are regarded to be close to the lepocreadiids and are nested in a clade named Lepocreadiata in a molecular study [39], as also adopted by Bray [40], who placed gyliauchenids in the Lepocreadioidea. The genus Robphildollfusium has been established as a gyliauchenid although its status is problematic [41]. These viewpoints could be supported by spermatological characters observed in some species belonging to the Lepocreadioidea. In fact, many similarities are observed between the spermatological characters of the two gyliauchenids and those of other lepocreadioid species, especially the lepocreadiid H. micracanthum [26]. This fact would allow us to support the inclusion of the Gyliauchenidae in the superfamily Lepocreadioidea. Concerning the problematic genus Robphildollfusium [41], similarities between the mature spermatozoon of R. fractum and Gyliauchen sp. could be valuable arguments to consider R. fractum as a gyliauchenid. However, more ultrastructural studies are required in the remaining gyliauchenid species and also in the other unexplored lepocreadioid families considering that, out of the ten families that compose this superfamily [40], only four have been studied until now.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
Authors wish to thank Núria Cortadellas and Almudena García from the “Unitat de Microscòpia, Facultat de Medicina, Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)” for their support in the preparation of samples. This study was partly supported by the PCI project (no A/030039/10) of the “Agencia Española de Cooperación Internacional para el Desarrollo (AECID)”. A.J.S. Bakhoum benefits from a MAEC-AECID doctoral grant (2010-11, no 0000538055).


