1 Introduction
During the last half-century, ultrastructural descriptions of spermatozoa were used as valuable tools for phylogenetic inference in the flatworms (Platyhelminthes), particularly in the tapeworms (Eucestoda) [1–10], but also in the Monogenea [4,11]. Considering digeneans, the increase of this kind of study has motivated the analysis of spermiological data, particularly those related to the mature spermatozoon, in order to establish different types of spermatozoa according to their ultrastructural organization. In the future, the establishment of different types of spermatozoa may be useful for a better knowledge of digenean relationships [12–16]. However, few ultrastructural investigations have been carried out on the superfamily Lepocreadioidea Odhner 1905. According to Bray [17], the Lepocreadioidea include ten families, namely the Lepocreadiidae, Acanthocolpidae, Apocreadiidae, Brachycladiidae, Deropristidae, Enenteridae, Gorgocephalidae, Gyliauchenidae, Liliatrematidae and Megaperidae. Nevertheless, based on molecular analyses, Bray et al. [18] and more recently Bray and Cribb [19] re-organized the superfamily Lepocreadioidea. Bray et al. [18] found the Lepocreadioidea to be monophyletic and constituted by six well-supported groups, which are presently considered families according to Bray and Cribb [19]. These families are the Lepocreadiidae, the Aephnidiogenidae and the Lepidapedidae, which were previously considered by Bray [20] as three subfamilies of the Lepocreadiidae s.l., and the Enenteridae, Gorgocephalidae and Gyliauchenidae. Moreover, according to Bray and Cribb [19], the families Acanthocolpidae, Apocreadiidae and Brachycladiidae are not closely related to the Lepocreadiidae and should be placed out of the Lepocreadioidea. With respect to the three remaining Lepocreadioidea families considered by Bray [17], namely the Deropristidae, Liliatrematidae and Megaperidae, molecular studies are still lacking. Among all these families, there are ultrastructural studies of the spermatozoon in the Aephnidiogenidae, Apocreadiidae, Deropristidae and Gyliauchenidae [14,21–24]. The present article presents the first assessment of the ultrastructural organization of the spermatozoon of a species belonging to the family Lepocreadiidae: Hypocreadium caputvadum. Our results are compared with those of other digeneans, particularly lepocreadioideans.
2 Materials and methods
Live specimens of H. caputvadum Kacem et al., 2011 [25] were collected from the intestine of grey triggerfish Balistes capriscus Gmelin, 1789 (Balistidae), caught in the Gulf of Gabès off Chebba (Tunisia) (34°14′N, 11°06′E). After dissection, live digeneans were routinely processed for TEM examination. Therefore, they were fixed in cold (4 °C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.4 for a minimum of 2 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.4, postfixed in cold (4 °C) 1% osmium tetroxide in the same buffer for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.4, dehydrated in an ethanol series and propylene oxide, and finally embedded in Spurr resin. Seminal vesicle was located in semi-thin sections. Later ultrathin sections were obtained using a Reichert-Jung Ultracut E ultramicrotome, placed on copper grids and double-stained with uranyl acetate and lead citrate according to Reynolds methodology [26]. Ultrathin sections were examined using a JEOL 1010 transmission electron microscope operated at an accelerating voltage of 80 kv at the CCiTUB (“Serveis Científics i Tecnològics” of the University of Barcelona, Spain).
The Thiéry technique [27] was used to locate glycogen. Gold grids were treated in periodic acid, thiocarbohydrazide and silver proteinate (PA-TCH-SP) as follows: 30 min in 10% PA, rinsed in milliQ water, 24 h in TCH, rinsed in acetic solutions and milliQ water, 30 min in 1% SP in the dark, and rinsed in milliQ water.
3 Results
The observation of numerous cross- and longitudinal sections allows us to distinguish three different regions from the anterior to the posterior extremities of the mature spermatozoon of H. caputvadum, each exhibiting distinctive ultrastructural characters (Figs. 1–3). The mature spermatozoon is a long filiform cell tapered at both ends and exhibiting the usual structures found in the great majority of digeneans. Thus, it contains two axonemes, external ornamentation of the plasma membrane, nucleus, mitochondria, two parallel bundles of cortical microtubules and granules of glycogen.
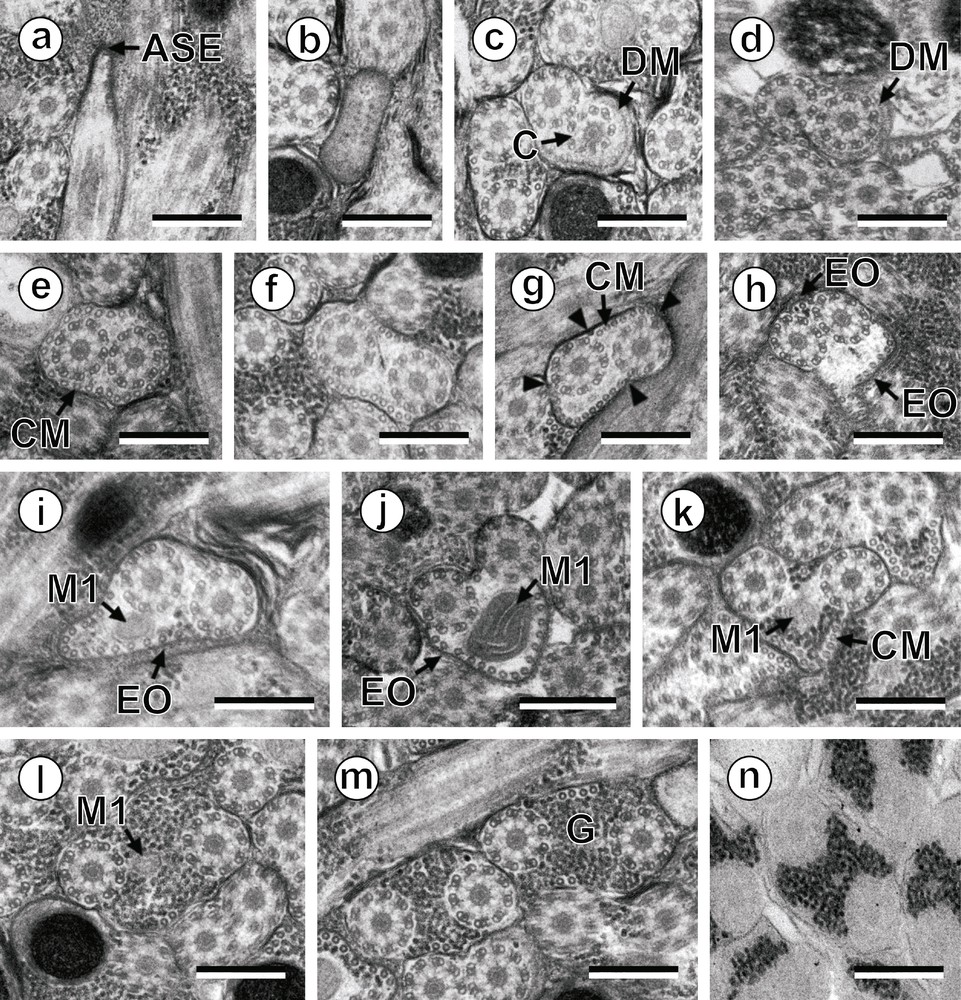
Prenuclear areas of the spermatozoon of Hypocreadium caputvadum: (a): longitudinal section of region I showing the anterior spermatozoon extremity; (b–m): consecutive cross-sections showing (i) the anterior spermatozoon tip (b), (ii) the formation of the second axoneme surrounded by an electron-dense material (c and d), (iii) areas exhibiting the maximum number of cortical microtubules (e and g), (iv) areas containing the external ornamentation of the plasma membrane (h–j) and the first mitochondrion (i–l), and (v) transitional areas before the appearance of the nucleus (m); (n): evidence of glycogen by a positive test of Thiéry. Scale bars: 0.3 μm. Arrowhead: attachment zones; ASE: anterior spermatozoon extremity; C: centriole; CM: cortical microtubules; DM: electron-dense material; EO: external ornamentation of the plasma membrane; G: granules of glycogen; M1: first mitochondrion.

Nuclear area of the spermatozoon of Hypocreadium caputvadum: (a–i): consecutive cross-sections of the nuclear area of the sperm cell showing (i) the appearance of the nucleus (a), (ii) the simultaneous presence of the nucleus and the second mitochondrion (b and c), (iii) the progressive disorganization of the first axoneme (d–f), (iv) the disorganization of the second axoneme and the disappearance of cortical microtubules (g and h), and (v) the posterior sperm tip containing only doublets and granules of glycogen (i). Scale bars: 0.3 μm. Ax: axoneme; CC: central core; CM: cortical microtubules; D: doublets; G: granules of glycogen; M2: second mitochondrion; N: nucleus.
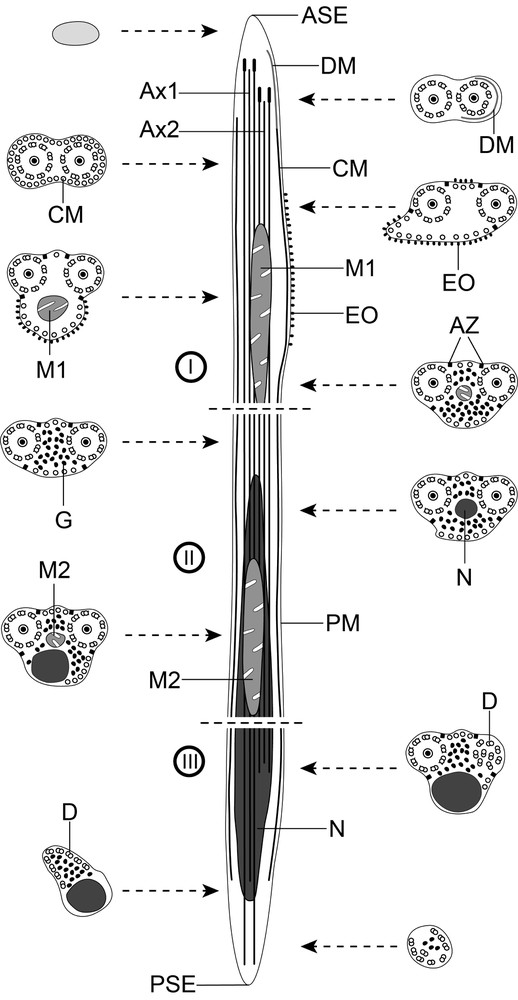
Schematic reconstruction of the mature spermatozoon of Hypocreadium caputvadum. To simplify the diagram, glycogen granules are not shown in the longitudinal section. ASE: anterior spermatozoon extremity; Ax: axoneme; Ax1: first axoneme; Ax2: second axoneme; AZ: attachment zones; C: centriole; CC: central core; CM; cortical microtubules; D: doublets; DM: electron-dense material; EO: external ornamentation of the plasma membrane; G: granules of glycogen; M1: first mitochondrion; M2: second mitochondrion; N: nucleus; PM: plasma membrane; PSE: posterior spermatozoon extremity.
Region I (Figs. 1a–n, 3I) corresponds to the anterior extremity of the spermatozoon. The anterior part of this region is filiform, devoid of axonemes and moderately electron-dense (Figs. 1a, b, 3I). The axonemes of the 9 + “1” trepaxonematan pattern are slightly longitudinally shifted (Figs. 1c, 3I). The second axoneme is partly surrounded by a discontinuous and submembranous layer of electron-dense material (Figs. 1c, d, 3I). Posteriorly, cortical microtubules appear surrounding both axonemes as a continuous layer (Figs. 1e, 3I). Cortical microtubules progressively become organized into two fields (Figs. 1f, g, 3I). In the middle part of this region, an external ornamentation of the plasma membrane is observed in association with cortical microtubules (Figs. 1h–j, 3I). In this area, the first mitochondrion appears (Figs. 1i, j, 3I). In the distal area of region I, we notice the absence of external ornamentation, the reduction in the size of the mitochondrion and the appearance of granules of glycogen (Figs. 1k, l, 3I). The posterior extremity of the first mitochondrion marks the transition towards the region II (Figs. 1l, 3I). The glycogenic nature of this granular material was evidenced by the test of Thiéry (Fig. 1n).
Region II (Figs. 1m, 2a–d, 3II) corresponds to the middle region of the spermatozoon, which is mainly characterized by the simultaneous presence of the second mitochondrion and the anterior part of the nucleus. Anterior areas of this region show two axonemes, cortical microtubules and granules of glycogen (Figs. 1m, 3II). At a slightly lower level, we notice the appearance of the nucleus (Figs. 2a, 3II). The distal area of region II exhibits the simultaneous presence of both nucleus and the second mitochondrion (Figs. 2b, c, 3II). Finally, region II ends at the posterior extremity of the second mitochondrion.
Region III (Figs. 2d–i, 3III) corresponds to the posterior region of the spermatozoon, which is characterized by the presence of the posterior part of the nucleus. Consecutive cross-sections show:
- • the presence of two axonemes, nucleus, cortical microtubules and granules of glycogen (Fig. 2d);
- • the disorganization of the first axoneme (Figs. 2e, f, 3III);
- • the disorganization of the second axoneme and disappearance of cortical microtubules (Figs. 2g, h, 3III);
- • the nucleus distal extremity followed by the complete disappearance of doublets from the second axoneme (Figs. 2h, i, 3III). The posterior spermatozoon tip exhibits doublets from the last axoneme and few granules of glycogen (Figs. 2i, 3III).
4 Discussion
The mature spermatozoon of H. caputvadum shows the usual ultrastructural elements observed in most digeneans so far: two axonemes, nucleus, mitochondria, granules of glycogen, external ornamentation of the plasma membrane and two bundles of parallel cortical microtubules.
It possesses two axonemes with different lengths exhibiting the classical 9 + “1” trepaxonematan pattern [28]. This is the typical structure of axonemes observed in all digeneans except for species of the genus Schistosoma with a special 9 + “1” pattern [29] and species of Didymozoon with a 9 + 0 pattern [30,31].
Concerning the anterior spermatozoon extremity, H. caputvadum exhibits both axonemes; however, to our knowledge, in the remaining studied species of the superfamily Lepocreadioidea, there is only one axoneme in their anterior tip as in the Gyliauchenidae Gyliauchen sp. and Robphildollfusium fractum [14,24] and the Aephnidiogenidae Holorchis micracanthum [23]. In the problematic families Apocreadiidae and Deropristidae there is also a single axoneme in the anterior spermatozoon extremity [21,22]. In this anterior spermatozoon extremity, there is also a particular feature that consists in a discontinuous electron-dense material partially surrounding the second axoneme beneath the plasma membrane. Within the lepocreadiodeans, this electron-dense material has been described in the three analysed families, namely the Aephnidiogenidae, Gyliauchenidae and Lepocreadiidae [14,23,24, present study].
The spermatozoon of H. caputvadum displays external ornamentation of the plasma membrane as occurs in the remaining lepocreadioideans studied to date [14,23,24] and also in the apocreadiids and deropristids [21,22]. The role of these elements remains unknown. Nevertheless, Justine and Mattei [32] hypothesized that external ornamentation participates in the fusion of the spermatozoon and ovum membranes during fertilization. In the digenean spermatozoon, the external ornamentation is present in anterior areas of the sperm cell and can present different locations. According to Quilichini et al. [14], there are three types of anterior spermatozoon regions depending on this character:
- • type 1 presents external ornamentation in the anterior extremity of the spermatozoon;
- • type 2 presents external ornamentation at a more posterior level, usually in the mitochondrial region;
- • type 3 lacks external ornamentation.
According to this classification, H. caputvadum is included in the second type.
The number of mitochondria in the spermatozoon of digeneans is a matter of controversy [12]. Traditionally, it was accepted that during spermiogenesis, several mitochondria fuse to form a unique and long mitochondrion present in the mature spermatozoon [33,34]. Nevertheless, in order to make a logical interpretation of their observations, several authors have described more than one mitochondrion. Thus, there are descriptions of digenean spermatozoa containing one, two or three mitochondria. In the spermatozoa of the Lepocreadioidea, both number and form of the mitochondrion are variable. Two mitochondria have been observed in the spermatozoon of the lepocreadiid H. caputvadum; the first one is located at the level of the external ornamentation of the plasma membrane and the second one is located in the area containing the nucleus. In the aephnidiogenid H. micracanthum Bâ et al. [23] described a moniliform mitochondrion that appears in the form of successive bulges, connected to each other by a mitochondrial cord, and it extends almost throughout the whole length of the spermatozoon. In the Gyliauchenidae, there are two studied species namely Gyliauchen sp. and R. fractum, that exhibit one and two mitochondria respectively [14,24]. Concerning the apocreadiids and deropristids, the mature spermatozoa of both Neoapocreadium chabaudi and Deropristis inflata present two mitochondria [21,22].
The posterior tip of digenean spermatozoa is morphologically variable. Quilichini et al. [13] distinguished three types of posterior parts of the spermatozoon (opecoelidean type, fasciolidean type and cryptogonimidean type). These types are characterized by the sequence of characters towards the posterior spermatozoon tip. According to these authors, there is a possibility of a fourth group characterized by a different sequence: posterior extremity of the first axoneme, posterior extremity of cortical microtubules and posterior extremity of the second axoneme. This group would be represented by the Deropristidae D. inflata [21], the Brachylaimidae Scaphiostomum palaearcticum [35] and the Lecithasteridae Aponurus laguncula [36]. In our study, the posterior spermatozoon extremity of H. caputvadum exhibits only a few doublets resulting from the disorganization of the second axoneme, and glycogen granules. So, H. caputvadum belongs to the cryptogonimidean type of Quilichini et al. [13]. On the other hand, taking into account several incongruences in the described posterior sperm types, several authors discussed the consideration of only the terminal character [37] instead of the sequence of characters towards the posterior spermatozoon tip. With respect to the remaining Lepocreadioidea, all the studied species exhibit the second axoneme as terminal character except R. fractum (Gyliauchenidae), which presents the nucleus extremity in the posterior spermatozoon tip [24].
Considering the recent reorganisation of the superfamily Lepocreadioidea [19] we summarize in Table 1 the most significant ultrastructural characters of the spermatozoon found in digeneans belonging to this group. We include in Table 1 the spermatological characters of the family Apocreadiidae (not related to lepocreadiids [19]) and also of the family Deropristidae, which lacks a molecular study confirming its status in the Lepocreadioidea. The unique distinguishing ultrastructural character present in the sperm cell is the electron-dense material surrounding one of the axonemes in the anterior spermatozoon extremity. This character is present in lepocreadiids, aephnidiogenids and gyliauchenids and it is absent in both apocreadiids and deropristids. In spite of the scarce ultrastructural studies on the superfamily, the presence of this character in the spermatozoa of these three families and its absence in apocreadiids demonstrates the utility of the sperm ultrastructure as a tool for phylogenetic inference in the Lepocreadioidea.
Ultrastructural characters of the spermatozoon in the Lepocreadioideaa and in the families Apocreadiidae and Deropristidae.
| Families and species | Spermatozoon characters | References | ||||
| ASE | EO | SB | M | PSE | ||
| Families included in the Lepocreadioidea a | ||||||
| Aephnidiogenidae | ||||||
| Holorchis micracanthum | Ax-DM | + | – | 1 | Ax | [23] |
| Gyliauchenidae | ||||||
| Gyliauchen sp. | Ax-DM | + | + | 1 | Ax | [14] |
| Robphildollfusium fractum | Ax-DM | + | + | 2 | N | [24] |
| Lepocreadiidae | ||||||
| Hypocreadium caputvadum | Ax-DM | + | – | 2 | Ax | Present study |
| Families not included in the Lepocreadioidea a | ||||||
| Apocreadiidae | ||||||
| Neoapocreadium chabaudi | Ax-EO-CM | + | + | 2 | N | [22] |
| Deropristidae | ||||||
| Deropristis inflata | Ax | + | – | 2 | Ax | [21] |
a Lepocreadioidea according to Bray and Cribb [19]. Only the families studied in the molecular analysis of these authors are considered.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to Núria Cortadellas and Almudena García from the “Unitat de Microscòpia, Facultat de Medicina, Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)” for their support in the preparation of samples. We are also grateful to R.A. Bray who provided a manuscript in press. This study was partly supported by the Spanish Projects A/015863/08 and A/023585/09 from the “Agencia Española de Cooperación Internacional para el Desarrollo (AECID), Ministerio de Asuntos Exteriores y de Cooperación (MAEC)”. A.J.S. Bakhoum benefits from MAEC-AECID doctoral grants (2009–10, no. 0000448019 and 2010–11, no. 0000538055). C. Eira was supported by a grant (SFRH/BPD/27014/2006) from the Portuguese Foundation for Science and Technology (FCT).


