1 Introduction
Trypanorhynchs are polyzoic cestodes, readily recognised by their rhyncheal apparatus, and are common metazoan parasites of marine fish. Adult worms parasitize elasmobranch fishes while their larval stages occur in a variety of teleosts, elasmobranchs and marine invertebrates (crustaceans, cephalopods and bivalves), including zooplankton [1]. Trypanorhynchs possess a scolex with two or four bothria [2] and also a tentacular apparatus consisting of four retractile tentacles armed with hooks that are attached to four bulbs [3]. This rhyncheal apparatus is unique within the cestodes, and provides a strong synapomorphy that supports the monophyly of this order [4].
This order has been considered to be one of the most chaotic and confusing tapeworm groups, but recent work has shed considerable light on their systematics [5]. Morphological evidence strongly supports the monophyly of trypanorhynchs [1,5]. Although Waeschenbach et al. [6] presented molecular data that support the monophyly of the Trypanorhyncha, other molecular studies have suggested that this order is paraphyletic and consists of two well-supported clades [4,7–9]. The first clade groups together the superfamilies Eutetrarhynchoidea and Tentacularioidea while the second clade groups together the Gymnorhynchoidea, the Lacistorhynchoidea and the Otobothrioidea. Recently, Olson et al. [10], in a combined analysis of molecular and morphological data, identified two clades with each one occurring in the two principal clades of the elasmobranch definitive hosts (rays or sharks) and they proposed the suborders Trypanobatoida and Trypanoselachoida for these two major clades according to the primarily hosts parasitized, rays and sharks, respectively. The Trypanobatoida contains the Tentacularioidea (including the eutetrarhynchoids) and the Trypanoselachoida contains the Lacistorhynchoidea, the Otobothrioidea and the Gymnorhynchoidea.
The ultrastructural studies on cestode spermatozoa have proved useful in interpreting their phylogenetic relationships within the Platyhelminthes and could, therefore, provide useful morphological indicators of the phylogeny and/or classification of trypanorhynchs [11–19]. However, in the Trypanorhyncha, studies of spermiogenesis and/or spermatozoa are limited to species belonging to three of the five superfamilies, namely Gymnorhynchoidea, Lacistorhynchoidea and Eutetrarhynchoidea. These are Grillotia erinaceus and Lacistorhynchus tenuis [20–22] (Lacistorhynchoidea), Aporhynchus menezesi [23] (Gymnorhynchoidea), and Dollfusiella spinulifera and Parachristianella trygonis [24,25] (Eutetrarhynchoidea).
The aim of this present study is to analyse, for the first time, the spermatological patterns of two species of the unexplored superfamily Tentacularioidea, Nybelinia queenslandensis and Kotorella pronosoma. The present study also provides supplementary data on the superfamily Eutetrarhynchoidea, with the analysis of spermiogenesis and mature spermatozoon of two progrillotiids, Progrillotia dasyatidis and Progrillotia pastinacae, and new ultrastructural data concerning spermiogenesis in the eutetrarhynchids Dollfusiella spinulifera and Parachristianella trygonis. These new ultrastructural observations are focussed in the number of plates, constituting the intercentriolar body during spermiogenesis and in the variability of the arc-like row of thick cortical microtubules present in the anterior areas of the spermatozoon. In fact, these two characters are showed as the most interesting in this order because of its variability according to the species.
2 Materials and methods
Live adult specimens of N. queenslandensis and K. pronosoma were collected by Prof. Ian Beveridge, University of Melbourne, from Carcharhinus melanopterus and Himantura granulata, respectively, caught off Lizard Island (Queensland, Australia). D. spinulifera was provided by Prof. Malcolm K. Jones from Rhinobatos typus collected on the reef flats at Heron Island (Queensland, Australia). Pro. dasyatidis and Pro. pastinacae were collected from Dasyatis tortonesei and Dasyatis pastinaca, respectively, caught off Sidi Mansour and Zarzis (Gulf of Gabès, Tunisia). Finally, P. trygonis was collected from Dasyatis pastinaca caught off Sidi Mansour (Gulf of Gabès, Tunisia). All the three species isolated from Tunisian elasmobranchs were collected in collaboration with Dr Lassad Neifar (University of Sfax).
After dissection, the mature proglottids from these cestodes were routinely processed for transmission electron microscopy examination. Thus, they were fixed in cold (4 °C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.4 for a minimum of 2 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.4, post-fixed in cold (4 °C) 1% osmium tetroxide (OsO4) with 0.9% potassium ferricyanide [K3Fe(CN)6] in the same buffer for 1 h, rinsed in milliQ water, dehydrated in an ethanol series and propylene oxide, and finally, embedded in Spurr's resin. Ultrathin sections (50–60 nm thick) were obtained using a Reichert–Jung Ultracut E ultramicrotome, placed on copper grids and double-stained with uranyl acetate and lead citrate. The ultrathin sections were examined using a JEOL 1010 TEM operated at an accelerating voltage of 80 kV.
3 Results
3.1 Spermiogenesis
Spermiogenesis in all the species studied begins with the formation of a differentiation zone in each spermatid. This area is a conical-shaped region bordered by cortical microtubules and delimited at its base by a ring of arched membranes. It also has two centrioles associated with striated rootlets and separated by an intercentriolar body. The intercentriolar body consists of three electron-dense plates separated by electron-lucent ones in all the species studied (K. pronosoma, N. queenslandensis, D. spinulifera, P. trygonis, Pro. dasyatidis and Pro. pastinacae) ((Figs. 1–5)). The two centrioles develop into two orthogonal flagella with respect to a developing median cytoplasmic process. The cortical microtubules lengthen along this growing median cytoplasmic process (Fig. 6). The nucleus initiates its elongation and the two flagella undergo a flagellar rotation and become parallel to the median cytoplasmic process (Fig. 6). The flagellar rotation is followed by the proximo-distal fusion of the two flagella with the median cytoplasmic process. Finally, the ring of arching membranes is tightened and the young spermatozoon is detached from the residual cytoplasm.
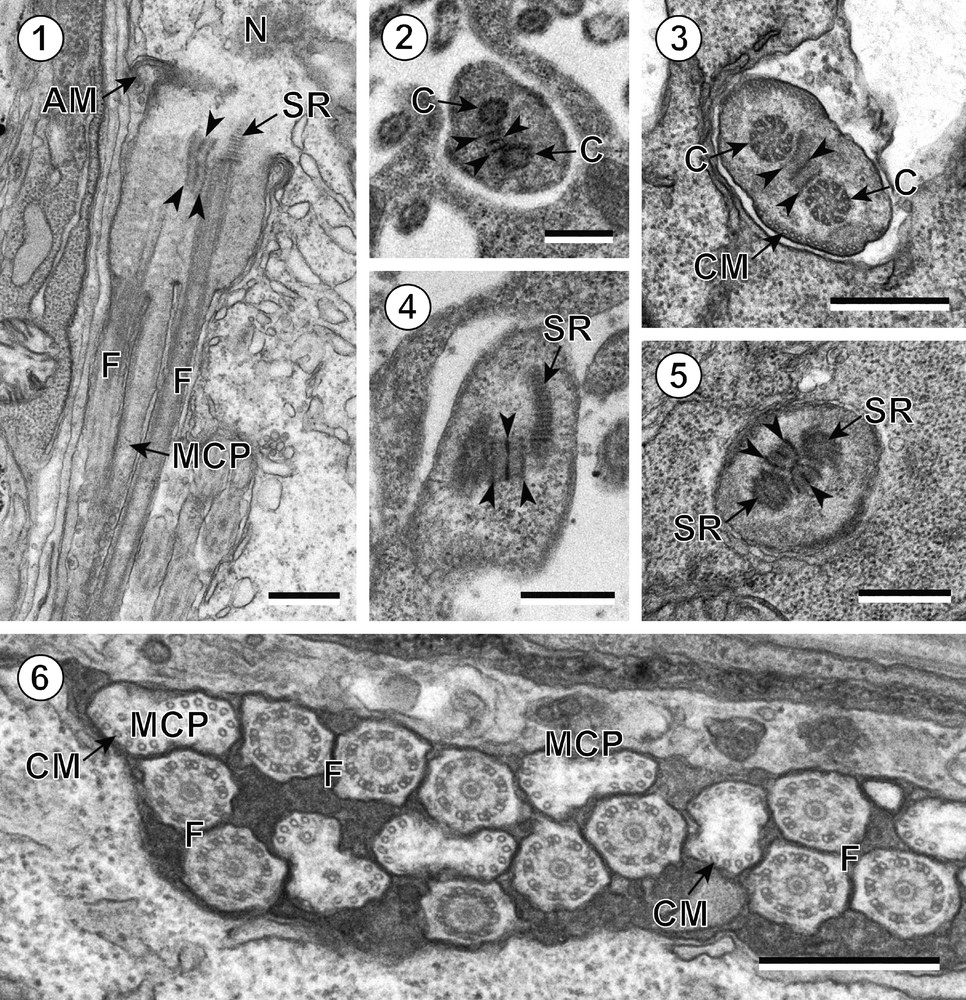
Spermiogenesis in trypanorhynchs. (1) Zone of differentiation of Parachristianella trygonis before the proximo-distal fusion of the flagella (F) with the median cytoplasmic process (MCP). Note the three electron-dense bands of the intercentriolar body (arrowheads). AM: arched membranes; N: nucleus; SR: striated rootlets. (2–5) Cross-sections showing the number of electron-dense plates of the intercentriolar body (arrowheads) in Nybelinia queenslandensis (Fig. 2), Dollfusiella spinulifera (Fig. 3), Kotorella pronosoma (Fig. 4) and Progrillotia dasyatidis (Fig. 5). C: centrioles; CM: cortical microtubules; SR: striated rootlets. (6) Cross-sections of flagella (F) and median cytoplasmic processes (MCP) before the proximo-distal fusion in Dollfusiella spinulifera. CM: cortical microtubules. Scale bars = 0.5 μm. Masquer
Spermiogenesis in trypanorhynchs. (1) Zone of differentiation of Parachristianella trygonis before the proximo-distal fusion of the flagella (F) with the median cytoplasmic process (MCP). Note the three electron-dense bands of the intercentriolar body (arrowheads). AM: arched membranes; N: nucleus; ... Lire la suite
3.2 Spermatozoon
The spermatozoon of trypanorhynchs, as in other cestodes, is a long filiform cell, tapered at both ends, which lacks mitochondria. Its cytoplasm contains: (1) two axonemes of different lengths with the 9 + ‘1′ pattern of trepaxonematan Platyhelminthes, (2) an arched row of thick and parallel cortical microtubules near the anterior extremity, (3) two rows of thin and parallel cortical microtubules, (4) a parallel nucleus, and (5) glycogen.
According to these ultrastructural characteristics, the mature spermatozoon of trypanorhynchs can be subdivided into four arbitrary regions characterized by different ultrastructural features.
Region I (Figs. 7–11) constitutes the anterior extremity of the sperm cell and contains a single axoneme. The anterior tip of the spermatozoon exhibits only a few microtubules. When the axoneme appears, it is surrounded by a submembraneous arc of thick and parallel cortical microtubules. In some species, several isolated cortical microtubules are located between this arc-like layer and the axoneme (Table 1).
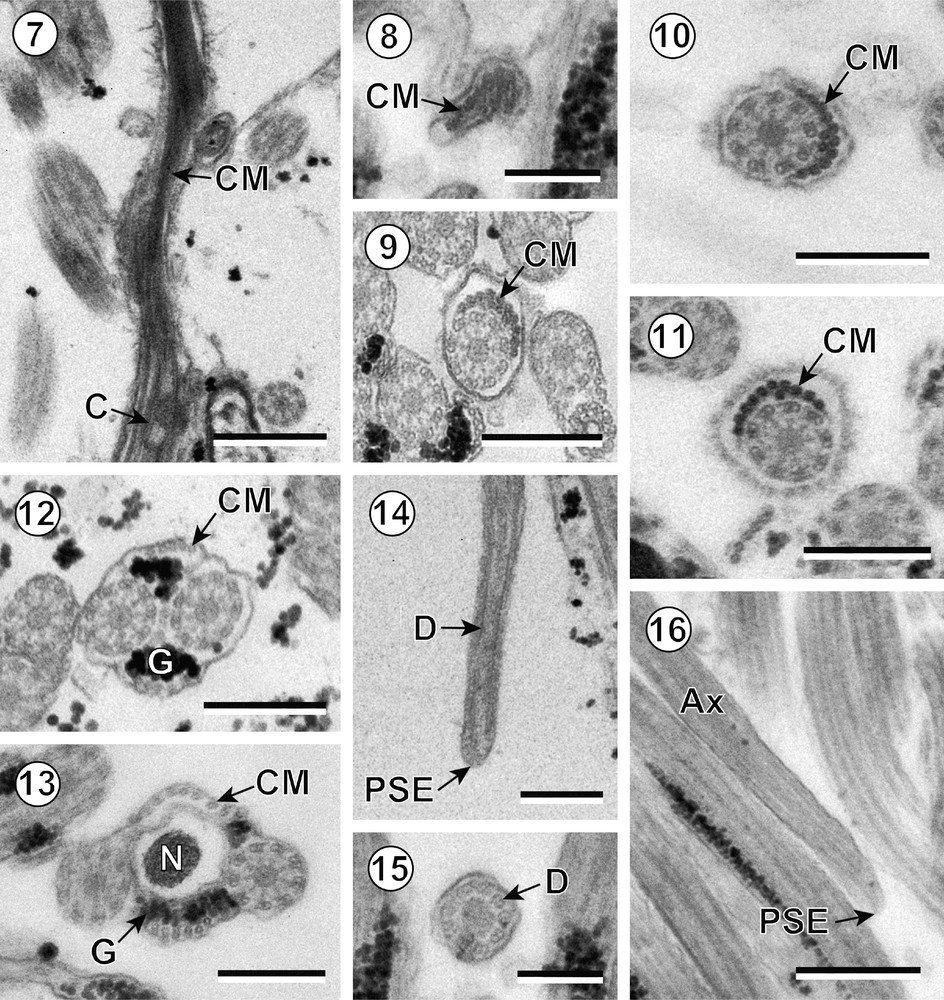
Mature spermatozoon in trypanorhynchs. (7) Longitudinal section near the anterior extremity of the spermatozoon of Kotorella pronosoma. C: centriole; CM: cortical microtubules. (8) Cross-section of the anterior extremity of the spermatozoon of Progrillotia pastinacae. CM: cortical microtubules. (9–11) Cross-sections of anterior areas of the spermatozoon (region I) of Kotorella pronosoma (Fig. 9), Progrillotia pastinacae (Fig. 10) and Nybelinia queenslandensis (Fig. 11) showing the arc-like row of thick cortical microtubules (CM). (12) Cross-section of region II of the spermatozoon of Kotorella pronosoma showing the two fields of thin cortical microtubules (CM) and granules of glycogen (G). (13) Cross-section of nuclear area (region III) of the spermatozoon of Progrillotia pastinacae showing the parallel disposition of the nucleus (N). CM: cortical microtubules; G: granules of glycogen. (14–16) Cross- and longitudinal sections of region IV of the spermatozoon of Kotorella pronosoma (Fig. 14) and Progrillotia pastinacae (Figs. 15–16) showing the posterior spermatozoon extremity (PSE) and the disorganization of the axoneme (Ax) into doublets (D). Scale bars = 0.5 μm (Figs. 7 and 16), 0.3 μm (Figs. 9–14), 0.2 μm (Figs. 8 and 15). Masquer
Mature spermatozoon in trypanorhynchs. (7) Longitudinal section near the anterior extremity of the spermatozoon of Kotorella pronosoma. C: centriole; CM: cortical microtubules. (8) Cross-section of the anterior extremity of the spermatozoon of Progrillotia pastinacae. CM: cortical microtubules. (9–11) ... Lire la suite
Ultrastructural characters of spermiogenesis and the spermatozoon in the trypanorhynchs.
| Superfamilies, families and species | Spermiogenesis | Spermatozoon | |||||||||||
| Typea | IB | Typeb | ASE | Ax | CB | ArcCM (n) | M | CM | N | G | PSE | Reference | |
| Tentacularioidea | |||||||||||||
| Tentaculariidae | |||||||||||||
| Kotorella pronosoma | I | 3 | I | CM | 2 | – | + (10 + 3) | – | 0° | 0° | + | 1Ax | [Present study] |
| Nybelinia queenslandensis | I | 3 | I | ? | 2 | – | + (10 + 1) | – | 0° | 0° | + | 1Ax | [Present study] |
| Gymnorhynchoidea | |||||||||||||
| Aporhynchidae | |||||||||||||
| Aporhynchus menezesi | I | 5 | I | CM | 2 | – | + (7) | – | 0° | 0° | + | 1Ax | [23] |
| Lacistorhynchoidea | |||||||||||||
| Lacistorhynchidae | |||||||||||||
| Grillotia erinaceus | I | 7 | I | ? | 2 | – | ? | – | 0° | 0° | + | ? | [20] |
| Lacistothynchus tenuis | I | 5 | I | ? | 2 | – | ? | – | 0° | 0° | + | ? | [21,22] |
| Eutetrarhynchoidea | |||||||||||||
| Eutetrarhynchidae | |||||||||||||
| Dollfusiella spinulifera | I | 3 | I | CM | 2 | – | + (10) | – | 0° | 0° | + | 1Ax | [24, present study] |
| Parachristianella trygonis | I | 3 | I | CM | 2 | – | + (10) | – | 0° | 0° | + | 1Ax | [25, present study] |
| Progrillotiidae | |||||||||||||
| Progrillotia dasyatidis | I | 3 | I | ? | 2 | – | + (?) | – | 0° | 0° | + | 1Ax | [Present study] |
| Progrillotia pastinacae | I | ? | I | CM | 2 | – | + (10 + 2) | – | 0° | 0° | + | 1Ax | [Present study] |
Region II (Fig. 12) is the pre-nuclear area containing two axonemes. In this region, the parallel cortical microtubules are thin and arranged in two fields.
Region III (Fig. 13) is the nuclear area of the spermatozoon. The nucleus is elongated and is disposed between the two axonemes. At the end of this region, the cortical microtubules disappear.
Region IV (Figs. 14–16) is the post-nuclear area of the male gamete and constitutes the posterior extremity of the spermatozoon that terminates with a single axoneme.
4 Discussion
4.1 Spermiogenesis
Spermiogenesis in trypanorhynchs is of type I of Bâ and Marchand [7]. This pattern is characterized by the growth of two orthogonal flagella with respect to a developing median cytoplasmic process. The two flagella undergo a flagellar rotation and become parallel to the median cytoplasmic process. Later, they fuse in a proximo-distal pattern and produce a spermatozoon with two axonemes.
The zone of differentiation is a conical-shaped region that contains two centrioles associated to two striated rootlets and exhibits an intercentriolar body between them. The intercentriolar body consists of a variable number of electron-dense plates according to the species. In our study we present new data on six species, namely D. spinulifera, P. trygonis, Pro. dasyatidis and Pro. pastinacae (Eutetrarhynchoidea), and K. pronosoma and N. queenslandensis (Tentacularoidea). In all of these species except for Pro. pastinacae, the intercentriolar body consists of three electron-dense plates. These are the species belonging to the Tentacularioidea and Eutetrarhynchoidea (Table 1). In previous studies, Świderski [22] describes seven electron-dense plates in Lacisthorhynchus tenuis, and McKerr [20] and Marigo et al. [23] describe five electron-dense plates in Grillotia erinaceus and Aporhynchus menezesi, respectively. These three species are included in two other superfamilies, namely the Gymnorhynchoidea and the Lacistorhynchoidea (Table 1).
4.2 Spermatozoon
The spermatozoon of trypanorhynchs, as in other cestodes, is a long filiform cell, tapered at both ends, which lacks mitochondria. Its cytoplasm contains: (1) two axonemes of different lengths with the 9+‘1′ trepaxonematan pattern [26], (2) an arched row of thick, parallel cortical microtubules near the anterior extremity, (3) two rows of thin, parallel cortical microtubules, (4) an elongated nucleus parallel to axonemes, and (5) glycogen in the form of α-glycogen rosettes and/or β-glycogen particles. Thus, the anterior and posterior extremities of the spermatozoon exhibit cortical microtubules and a single axoneme, respectively (Fig. 17).
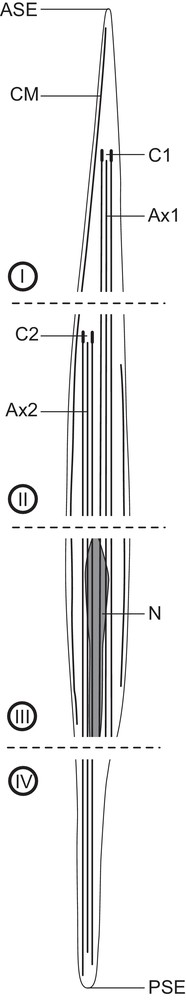
Schematic drawing of the mature spermatozoon of trypanorhynch species. ASE: anterior spermatozoon extremity; Ax1: first axoneme; Ax2: second axoneme; C1: centriole of the first axoneme; C2: centriole of the second axoneme; CM: cortical microtubules; N: nucleus; PSE: posterior spermatozoon extremity.
In fact, the ultrastructural organization of the mature spermatozoa in trypanorhynchs follows the type I of Levron et al. [19]. This pattern is also present in the orders Spathebothriidea, Diphyllobothriidea and Haplobothriidea and it is characterized by the presence of two axonemes and by the parallel disposition of both cortical microtubules and nucleus. The type I spermatozoon also lacks a crested body, a periaxonemal sheath and intracytoplasmic walls [19].
Unlike the majority of cestodes, trypanorhynch spematozoa lack a crested body or bodies [23-25, present study], and, consequently, the postulated synapomorphy of crested body or bodies for the Eucestoda is questionable [16,17,19]. There are other eucestode orders that also lack these anterior helical structures, e.g. the Caryophyllidea, the Spathebothriidea and the Diphyllobothriidea [27–38].
The arc-like row of cortical microtubules has been observed in the anterior part of the spermatozoon of numerous eucestodes, but there is variability in their aspect and number of elements. In trypanorhynchs the number of cortical microtubules forming this arc-like layer varies from seven to ten microtubules. Thus, K. pronosoma, N. queenslandensis, D. spinulifera, P. trygonis and Pro. pastinacae possess an arc-like row consisting of ten microtubules [24,25, present study], whereas according to Marigo et al. [23], in A. menezesi the maximum number is seven (Table 1). Anterior spermatozoon extremities showing a similar arrangement of cortical microtubules describing an arc-like layer are found in caryophyllideans [30,31,34,36,37], spathebothriideans [28,33], the former pseudophyllideans (Diphyllobothriidea and Bothriocephalidea) [29,32,39–43], the diphyllideans [44,45], the tetraphyllideans [46–50], the proteocephalideans [51–54], and in the mesocestoidid cyclophyllideans [55,56]. However, an arc-like layer consisting of thick cortical microtubules, as described in the trypanorhynchs, occurs only in the former pseudophyllideans, in the tetraphyllideans, in the proteocephalideans and in the mesocestoidids [29,32,39–42,46–56].
5 Concluding remarks
The results presented both here and in the previous studies indicate insignificant ultrastructural differences between the spermatological characters of the seven species studied and offer no additional support for the hypothesis, suggested by some authors that the trypanorhynchs are a polyphyletic group. However, these slight differences of sperm ultrastructure are coincident with the view of these authors [4,7–10]. The most remarkable differences between these species are (i) the number of electron-dense plates constituting the intercentriolar body (5 to 7 in the Gymnorhynchoidea and the Lacistorhynchoidea versus 3 in the Tentacularioidea and the Eutetrarhynchoidea) and (ii) the number of microtubules that form the arc-layer of thick cortical microtubules present in the anterior spermatozoon extremity (around 7 in the Gymnorhynchoidea and the Lacistorhynchoidea versus around 10 in the Tentacularioidea and the Eutetrarhynchoidea).
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors wish to thank Prof. Ian Beveridge (University of Melbourne, Australia) and Prof. Malcolm K. Jones (University of Queensland, Australia) for providing some of the specimens studied in the present work. The English of this manuscript was kindly corrected by Prof. Beveridge. We also thank Dr Lassad Neifar (University of Sfax, Tunisia) for their valuable help in the field work during Tunisian expeditions and also for identifying the specimens collected in Tunisia. Dr George McKerr (University of Ulster, North Ireland, UK) kindly provided a copy of his PhD thesis for comparative purposes. We are grateful to the “Unitat de Microscòpia, Facultat de Medicina, Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)” for their support in the preparation of samples, particularly Núria Cortadellas and Almudena García. The present study was partly funded by grants from the “Agencia Española de Cooperación Internacional para el Desarrollo (AECID)” (Nos. A/2390/05, A/6244/06, A/015863/08 and A/023428/09) to Jordi Miquel.


