1 Introduction
The scorpion genus Diplocentrus Peters, 1861 (Scopiones: Diplocentridae) is the most diverse in family Diplocentridae Karsch, 1880. This genus comprises nearly 60 species, most of which are endemic to Mexico [1–3]; however, it is distributed from southwestern USA (Arizona, Texas) to northern Honduras [4].
In Honduras, Diplocentrus is represented by three species [5]: D. coddingtoni Stockwell, 1988 (from north Honduras in Departmento Atlántida), D. lourencoi Stockwell, 1988 (from northwestern Honduras in Departamento Cortés) and D. santiagoi Stockwell, 1988 (from central western Honduras in Departamento Copán; Fig. 1). Scorpions are the best-studied order of arachnids in Honduras, but doubtless further discoveries remain. As part of ongoing studies, additional specimens of each known Honduran Diplocentrus have recently been re-collected from type localities (Santibáñez-López, in prep), plus further specimens from substantial collection efforts of diverse arachnids in Parque Nacional Cusuco, which as part of the Merendón mountains includes the type locality of D. lourencoi. Yet beyond limited studies at the above type localities, there have been very few arachnid studies in Honduras. In particular, the arachno-fauna of the north coast and the Islas de la Bahía are in urgent need of further research due to increasing pressure from human development. This coastal region of Honduras has long been associated with human development in recorded history through trade settlements and plantations, and more recently, an increased pressure from tourism.
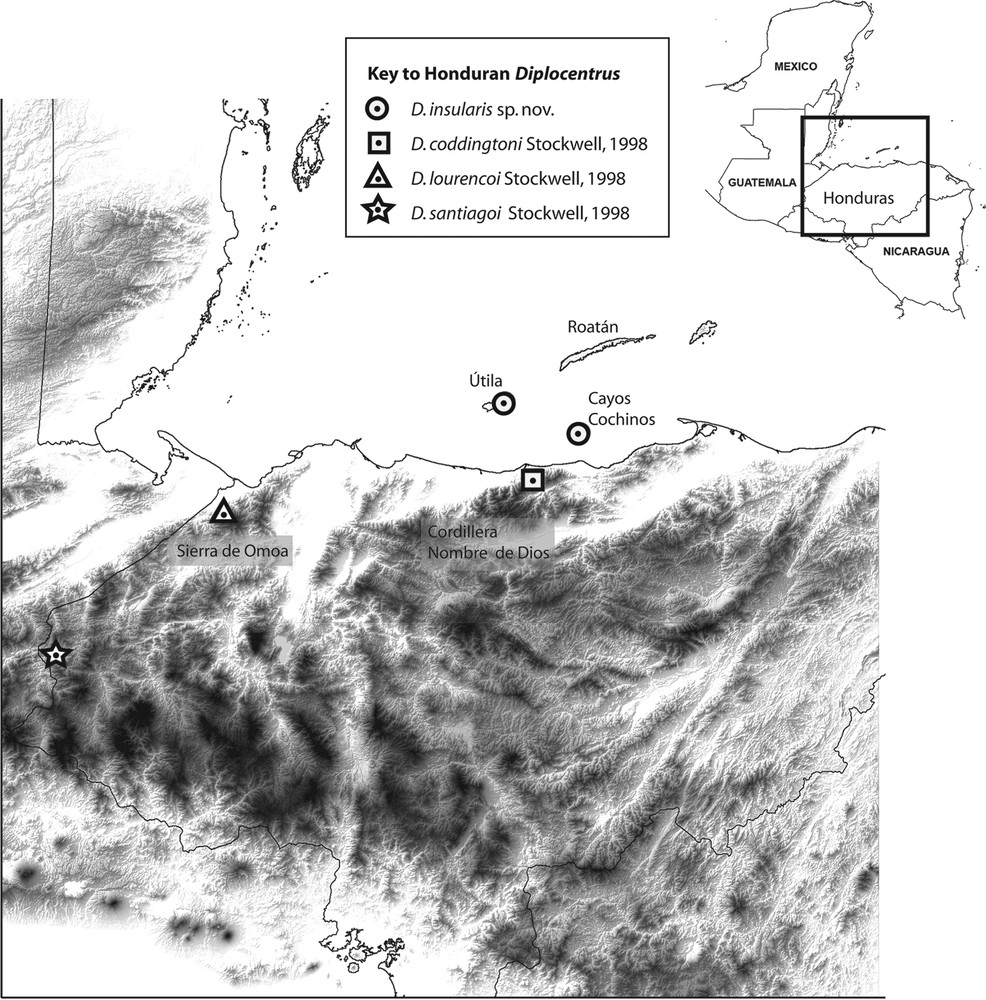
Diplocentrus insularis n. sp., known records in northern Honduras (in circles). Diplocentrus coddingtoni (type locality in square). Diplocentrus lourencoi (type locality in triangle). Diplocentrus santiagoi (type locality in star).
Scorpions are a diverse group on islands, especially in the Caribbean region, and diplocentrids are amongst the most studied (e.g., [6,7]). Three diplocentrid genera are endemic to Caribbean islands: Cazierus Francke, 1978, Cryptoiclus Teruel and Kovarik, 2012 and Oiclus Simon, 1880, while Heteronebo Pocock, 1899 is historically described from Abd-el-Kuri Island (Yemen) in the Saudi Arabia Peninsula, but with several later additions from the Greater Antilles [8]. Didymocentrus Kraepelin, 1905 is also endemic to the Caribbean region, but two species [D. krausi Francke, 1978 and D. nitidus (Hirst, 1907)] are restricted to Central America in El Salvador, Honduras, Nicaragua and Costa Rica [5,9,10]. The North American Bioculus Stahnke, 1968 also is represented by two island endemics in Baja California Sur: B. cerralvensis Stahnke, 1968 and B. cruzensis Stahnke, 1968 [11]; while B. comondae Stahnke, 1968 has some island populations, and two species are restricted to mainland Mexico [B. caboensis (Stahnke, 1968) in Baja California Sur, and B. parvulus Martín-Frías, 2004 in Guerrero]. While Diplocentrus is the most diverse genus in its family, until now only one species was known to be on an island endemic, namely D. cozumel Beutelspacher and Armas, 1998 (distributed across Cozumel island in southeast Mexico). This species is located almost 19 km away from its closest relatives in mainland Quintana Roo [2]; making this and the newly species described herein great candidates to study arachnid island colonization, plus important focal points for future conservation programs.
In the present contribution, we describe a fourth Honduran species of Diplocentrus from two islands in the northern Caribbean coast of Honduras, based on adult males and females. We compared it against its most similar species. A dichotomous key for the identification of species of Diplocentrus, and a map with known records in Honduras were also included.
2 Methods
Scorpions were collected during daytime and night time (without UV light detection), by turning rocks and logs, and under leaf litter.
The material is deposited in the following collections: American Museum of Natural History, New York (AMNH), with tissue samples stored in the Ambrose Monell Cryocollection (AMCC); Natural History Museum, London (BMNH), Colección Nacional de Arácnidos, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City (CNAN); Colección Entomologíca, Escuela Agrícola Panamericana “El Zamorano” (EPAZ), Hope Entomological Collection, Oxford University of Natural History (OUMNH), Museo de Historia Natural, Universidad Nacional Autónoma de Honduras (UNAH).
Geographical coordinates of collection localities were recorded in the field with (on Útila) a Garmin eTrex Venture or (on Cayos) an eTrex HCx GPS device. Distribution maps were generated in ArcView Ver. 3.2 (ESRI), using the locality coordinates, a base map from the ArcView Ver. 3.2 Database, and a digital elevation model from the CGIAR Consortium for Spatial Information [12].
Observations were made using Nikon SMZ-800 and SMZ-1500 stereomicroscopes. Measurements, given in millimeters, were obtained with an ocular micrometer calibrated at 10 ×. Hemispermatophores were dissected following Vachon [13], and cleared with pancreatin. Digital images were taken under visible and UV light with a Nikon SMZ-800 with Nikon Coolpix S10 VR camera attachment. The focal planes of image stacks were fused with CombineZM [14] and composite images edited with Adobe Photoshop CS5.
The scorpion higher classification follows Prendini and Wheeler [15]. The nomenclature and mensuration follows Stahnke [16], except for hemispermatophore [17], trichobothria [18], carination of the metasoma [19] and pedipalps [20], carapace surfaces [21], and basitarsal spiniform setae counts [1–3,22].
3 Systematics
Family DIPLOCENTRIDAE Karsch, 1880
Genus Diplocentrus Peters, 1861
Diplocentrus insularis n. sp.

(Color online.) Live habitus of Diplocentrus insularis n. sp. (A) ♂, (B) ♀.
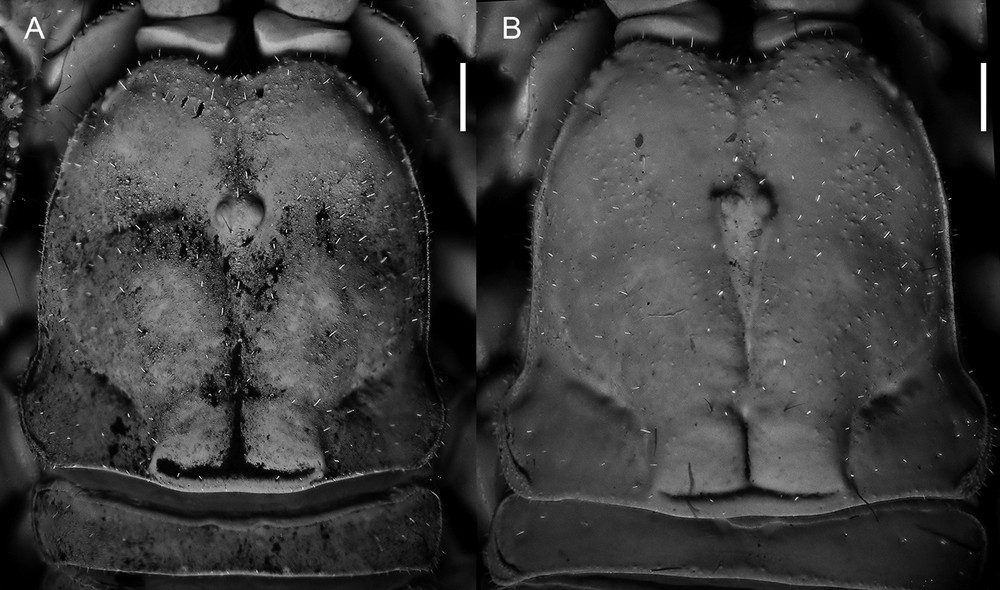
Diplocentrus insularis n. sp., carapace dorsal aspect. A. Holotype ♂ (CNAN-T0000). B. Paratype ♀ (CNAN-T0918). Scale bars = 1 mm.
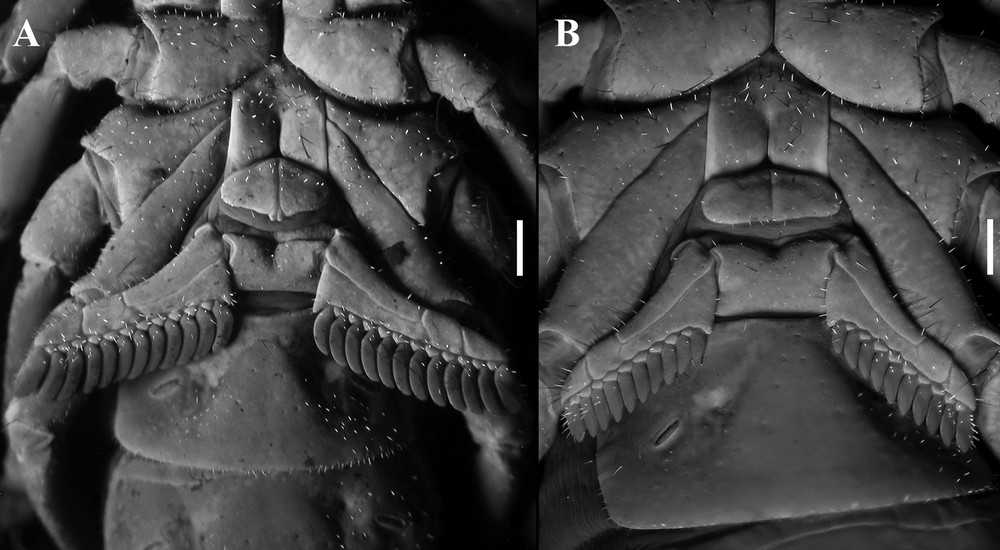
Diplocentrus insularis n. sp., sternum, genital operculum and pectines, ventral aspect. A. Holotype ♂ (CNAN-T0918). B. Paratype ♀ (CNAN-T0919). Scale bars = 1 mm.
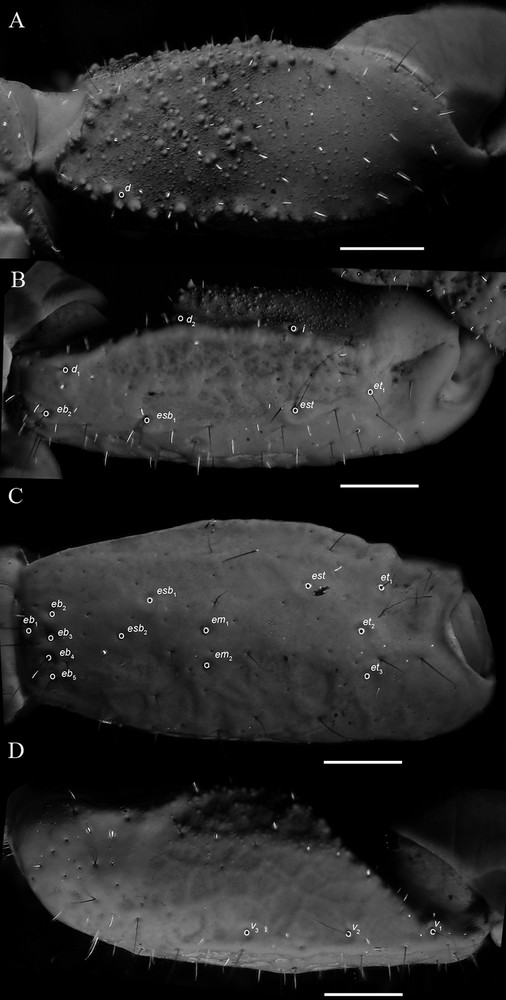
Diplocentrus insularis n. sp., holotype ♂ (CNAN-T0918), right pedipalp femur, dorsal aspect (A) and patella, dorsal aspect (B), retrolateral aspect (C), and ventral aspect (D), illustrating carinae and trichobothria. Scale bars = 1 mm. Abbreviations: d = dorsal; eb = external basal; em = external medial; esb = external subbasal; est = external subterminal; et = external terminal; I = internal; v = ventral.
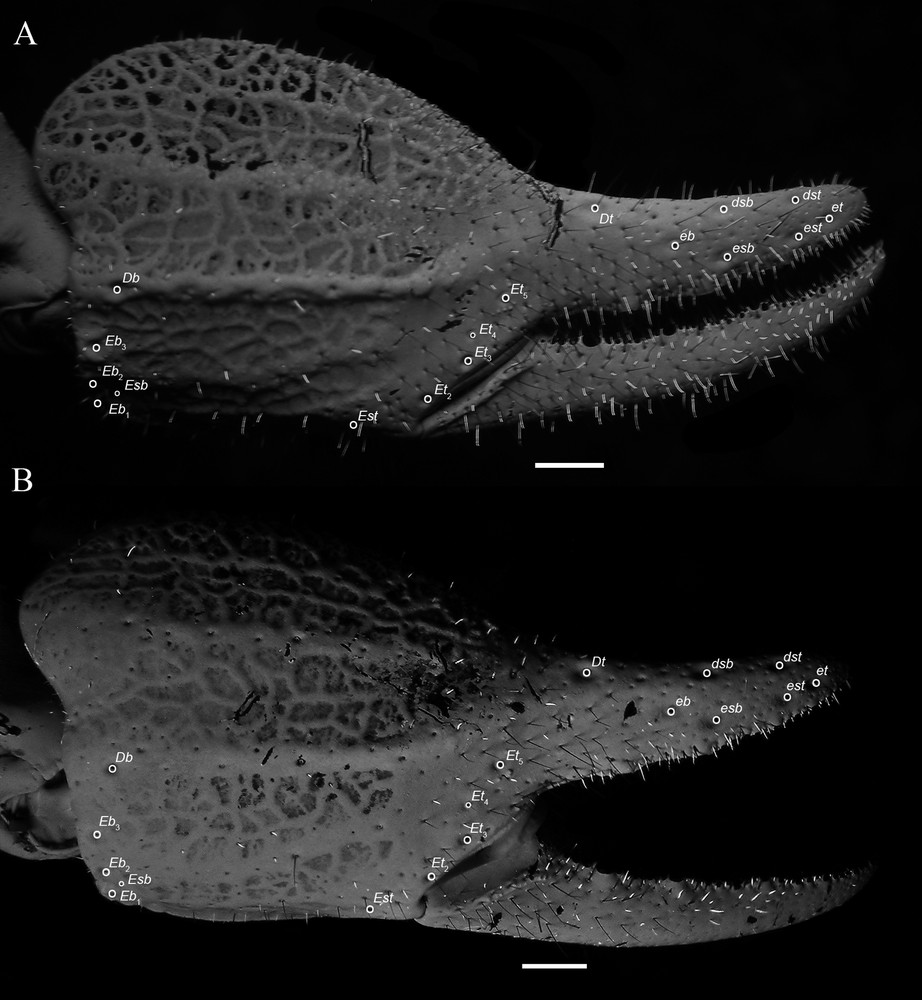
Diplocentrus insularis n. sp., right pedipalp chela, retrodorsal aspect. A. Holotype ♂ (CNAN-T0918). B. Paratype ♀ (CNAN-T0919), illustrating carinae and trichobothria. Scale bars = 1 mm. Abbreviations: Db = dorsal basal; dsb = dorsal subbasal; dorsal subterminal; Dt = dorsal terminal; Eb, eb = external basal; Esb, esb = external subbasal; Est, est = external subterminal; Et, et = external terminal.
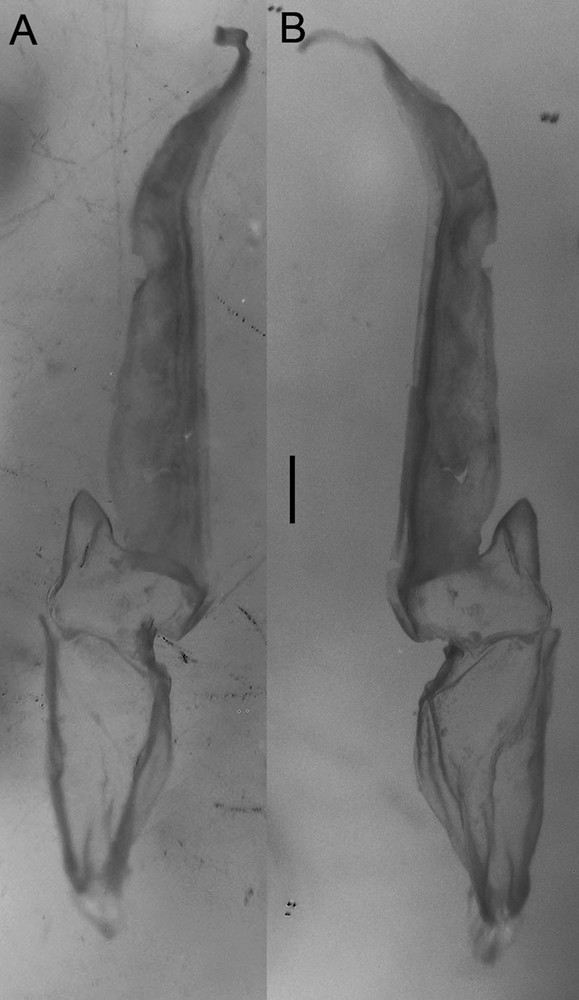
Diplocentrus insularis n. sp., paratype ♂ (CNAN-T0919) right hemispermatophore. A. Dorsal aspect. B. Ental aspect.

(Color online.) Habitat and microhabitat of Diplocentrus insularis n. sp. A–B. Type locality at Cayo Menor, Cayos Cochinos. C–D. Locality at Útila.
Measurements (mm) of male and female type specimens of Diplocentrus insularis, sp. n., in the CNAN.
| Diplocentrus insularis n. sp. | ||||
| ♂ | ♀ | |||
| Holotype | Paratype | |||
| CNAN-T0918 | CNAN-T0919 | |||
| Total | Length | 53.3 | 52.9 | |
| Carapace | Length | 6.3 | 6.9 | |
| Width | 5.4 | 5.8 | ||
| Mesosoma | Length | 17.1 | 17.5 | |
| Pedipalp | Length | 18.8 | 22.9 | |
| Femur | Length | 5.2 | 4.9 | |
| Width | 2.2 | 2.1 | ||
| Height | 2.0 | 2.3 | ||
| Patella | Length | 6.0 | 5.9 | |
| Width | 2.4 | 2.4 | ||
| Height | 2.5 | 2.8 | ||
| Chela | Length | 11.2 | 12.1 | |
| Width | 3.0 | 3.4 | ||
| Height | 5.4 | 6.2 | ||
| Movable finger | Length | 6.7 | 7.5 | |
| Fixed finger | Length | 4.8 | 5.0 | |
| Chelicera | Length | 3.7 | 4.2 | |
| Width | 1.5 | 1.7 | ||
| Movable finger | Length | 1.7 | 2.1 | |
| Fixed finger | Length | 1.3 | 1.5 | |
| Metasoma | Length | 23.9 | 22.6 | |
| Segment I | Length | 3.7 | 3.4 | |
| Width | 3.5 | 3.9 | ||
| Segment II | Length | 4.0 | 3.9 | |
| Width | 3.1 | 3.7 | ||
| Segment III | Length | 4.2 | 4.0 | |
| Width | 3.0 | 3.5 | ||
| Segment IV | Length | 5.0 | 4.8 | |
| Width | 2.8 | 3.2 | ||
| Segment V | Length | 7.0 | 6.5 | |
| Width | 2.5 | 2.5 | ||
| Height | 2.0 | 2.5 | ||
| Telson | Length | 6.0 | 5.9 | |
| Vesicle | Length | 4.7 | 4.5 | |
| Width | 2.5 | 3.4 | ||
| Height | 2.1 | 2.5 |
Telotarsal spiniform macrosetal count (number of macrosetae in pro- and retroventral rows of telotarsi on legs I–IV) in Diplocentrus insularis n. sp., given as the number of legs observed, with corresponding proventral (p) and retroventral (r) setal counts.
| Leg I | Leg II | Leg III | Leg IV | |||||||||||||||
| p | r | p | r | p | r | p | r | |||||||||||
| 3 | 4 | 3 | 4 | 5 | 3 | 4 | 3 | 4 | 5 | 4 | 5 | 5 | 5 | 4 | 5 | 6 | 7 | |
| D. insularis n. sp. | 1 | 65 | 1 | 63 | 2 | 1 | 65 | 1 | 43 | 22 | 1 | 60 | 64 | 62 | 2 | 56 | 4 | 1 |
Basitarsal spiniform macrosetal pattern (number of macrosetae of basitarsi on legs I–IV) in Diplocentrus insularis n. sp., given as numbers of legs observed, with corresponding setae count.
| Leg | n | pt | rt | vt | pst | rst | vst | pm | rm | vm | Rm |
| I | 60 | 8 | 0 | 0 | 60 | 60 | 0 | 59 | 49 | 0 | 51 |
| II | 60 | 58 | 57 | 0 | 58 | 58 | 0 | 55 | 54 | 0 | 58 |
| III | 56 | 56 | 56 | 56 | 0 | 20 | 56 | 0 | 0 | 53 | |
| IV | 55 | 55 | 55 | 55 | 0 | 23 | 55 | 0 | 0 | 52 |
Pectinal tooth count (number of teeth per pecten) in Diplocentrus insularis sp. n., given as number of male and female pectines observed, with corresponding tooth count.
| 9 | 10 | 11 | 12 | 13 | |
| D insularis n. sp. | |||||
| Male | 0 | 0 | 6 | 29 | 6 |
| Female | 1 | 18 | 3 | 0 | 0 |
Type material.HONDURAS: ISLAS DE LA BAHÍA: Municipio de Roatán: Holotype ♂ (CNAN-T0918) Cayo Menor, Cayos Cochinos 15.9572°N 86.4998°W, 8.viii.2012, K. Sgastume; 3 ♂, 1 ♀, 2 juv. paratypes (CNAN-T0919), 3 ♂, 1 ♀, 2 juv. paratypes (AMNH), 2 ♂ paratypes (OUMNH), 1 ♂ paratype (BMNH), 2 ♂ paratypes (UNAH), 1 ♂ paratype (EPAZ), same locality, 2-8.viii.2012, K. Sagastume.
Additional material. HONDURAS: ISLAS DE LA BAHÍA: Municipio de Roatán: 1 ♂, 1 ♀, 1 juv. (OUMNH), 1 ♂ (BMNH), 1 ♂, 1 ♀. (UNAH), 2 ♂, 1 ♀ (CNAN), 2 ♂ (AMNH), same locality as paratypes, 9–13.viii.2012, S. Longhorn and K. Sagastume. Municipio de Útila: 1 ♂ subad., 1 ♀ paratypes, 1 juv. (CNAN), 1 ♀ (AMNH), 1 ♀ (OUMNH), 2 ♀ (UNAH) New Airport forest patch, Útila 16.2034°N 86.8833°W, 14-27.vii.2012, K. Sagastume.
Etymology. The specific epithet refers to the fact that this species appears to be an island endemic. It is neutral in gender.
Diagnosis. The following character combination is diagnostic for D. insularis, n. sp. Total length (adult), 50–54 mm. Base coloration (adult), brown to reddish brown. Carapace anteromedian notch moderately deep, U-shaped (Fig. 3). Pedipalp femur, width greater than height, dorsal surface flat (Fig. 5A). Pedipalp patella, dorsal median carina weakly developed, smooth (♂, Fig. 5B); dorsal retrolateral carina weakly to moderately developed, smooth; retrolateral median carina obsolete (♂; ♀); ventral median carina weakly to moderately developed, smooth (Fig. 5D). Pedipalp chela manus, dorsal intercarinal surface moderately reticulated (♂, ♀ Fig. 6); digital carina strongly developed, smooth (♂), or weakly to moderately developed, smooth (♀); dorsal secondary carina weakly to moderately developed, smooth to crenulated (♂) or weakly developed to obsolete, smooth (♀). Legs I–IV telotarsi, counts of spiniform macrosetae in pro- and retroventral rows, 4/4:4/4–5:5/5:5/5; basitarsi spiniform macrosetae pattern: leg I pst, rst, pm, rm, Rm; leg II pt, rt, pst, rst, pm, rm, Rm; legs III–IV pt, rt, vt, vst, vm. Pectinal tooth count, 11–13 (♂) mode = 12 or 10 mode = 10 (♀).
D. insularis n. sp. resembles D. lourencoi Stockwell, 1988 and D. coddingtoni Stockwell, 1988 in similar adult size and body coloration, and D. maya Francke, 1977 in similar telotarsi spiniform macrosetae count. It can be distinguished by the following. The telotarsi spiniform macrosetae counts on legs I–II are lower in D. insularis n. sp. (4/4:4/5) than in D. lourencoi and in D. coddingtoni (4/5: 5/5). The pedipalp chela manus (♂) is rounded in D. insularis, but slender in D. maya, D. lourencoi and D. coddingtoni. The pedipalp patella dorsal retrolateral carina is weakly to moderately developed, smooth in D. insularis, but moderately developed, slightly granular in D. maya and in D. lourencoi, or obsolete D. coddingtoni. The Rm spiniform macrosetae on basitarsi of leg I is present in D. insularis, but absent in D. coddingtoni, D. maya and D. lourencoi.
Description. Based on holotype ♂ and paratype ♀ (Fig. 2A) with differences in paratype ♀ (Figs. 2B) noted. Measurements in Table 1.
Coloration. Carapace medium to light brown, with weak fuscosity throughout, uniform around median eyes and variegated elsewhere; coxosternal region pale orange to pale brown. Pedipalps brown to orange–brown, carinae darker. Mesosoma medium brown (♂) to orange–brown (♀), ventral surface pale brown, tergites light to medium brown, with moderately dense variegated fusco-piceous pattern; sternites medium brown to pale brown. Metasoma reddish brown to pale brown. Telson orange brownish or reddish brown, uniformly fuscous. Legs orange–brown, uniformly infuscate.
Carapace. Anterior margin moderate to sparsely setose, anteromedian notch strongly deep, V-shaped (Fig. 3). Frontal lobes and interocular surface weakly granular to smooth; other surfaces shagreened. Three pairs of subequal lateral ocelli.
Pedipalps. Orthobothriotaxic, Type C. Femur width greater than height (Fig. 5A); dorsal intercarinal surface flat to slightly convex, shagreened to sparsely granular; retrolateral intercarinal surface smooth; ventral intercarinal surface flat, slightly shagreened to minutely granular; prolateral intercarinal surface coarsely and minutely granular, with moderate-size dark granules; dorsal prolateral carina moderately developed, granular; dorsal retrolateral carina moderately developed, fading distally, densely granular proximally and smooth distally; ventral retrolateral carina obsolete; ventral prolateral carina moderately developed, granular proximally, becoming obsolete distally. Patella (Figs. 5B–D), dorsal, retrolateral and ventral intercarinal surfaces finely reticulated (♂) to smooth (♀); prolateral intercarinal surface weakly, sparsely granular distally; proximal tubercle moderately developed, bifurcate; dorsal prolateral carina weakly developed to obsolete, slightly granular; dorsal median carina weakly to moderately developed, smooth; dorsal retrolateral carina, weakly to moderately developed smooth (♂) or weakly to obsolete, smooth (♀); retrolateral median carina obsolete (♂, ♀); ventral retrolateral carina weakly developed, smooth; ventral median carina weakly to moderately developed, slightly granular (♂) to weakly developed to obsolete, smooth (♀); ventral prolateral carina weakly to moderately developed, granular, comprising small granules and fading posteriorly. Chela manus, slender, height subequal to width (♂) or rounded, height greater than width (♀), densely (♂) or sparsely (♀) setose; dorsal intercarinal surface strongly reticulated (♂; Fig. 6A; ♀; Fig. 6B); retrolateral intercarinal surface reticulated, prolateral surface with shallow longitudinal depression where chela rests against patella; dorsal marginal carina moderately developed, strongly granular; digital carina strongly developed, smooth (♂) or weakly to moderately developed, smooth (♀); dorsal secondary and retrolateral secondary carinae weakly to moderately developed, smooth to crenulated (♂) or weakly developed to obsolete, smooth (♀); ventral retrolateral carina weakly developed, smooth distally (♂) or weakly developed to obsolete, smooth (♀), becoming obsolete proximally; ventral median carina strongly developed, crenulated proximally, becoming obsolete distally, directed towards midpoint of movable finger articulation; ventral prolateral carina moderately developed, crenulated; prolateral dorsal carina weakly developed, slightly granular; prolateral median and prolateral ventral carinae weakly developed, slightly granular. Chela fixed finger gently curved; length longer (♂) or equal (♀) to femur length and patella length; dorsal surface smooth and densely setose proximally, retrolateral surface flat; prolateral surface shallowly concave.
Legs. Legs I–IV femora and tibiae, prolateral surfaces shagreened; telotarsi, counts of spiniform macrosetae in pro- and retroventral rows (dextral/sinistral), 4/4 4/4: 4/5 4/5: 5/5 5/5: 5/5 5/5 (holotype) (variation shown in Table 2); basitarsi spiniform macrosetae pattern: (holotype) leg I pst, rst, pm, rm, Rm; leg II pt, rt, pst, rst, pm, rm, Rm; leg III pt, rt, vt, rst, vst, vm; leg IV pt, rt, vt, vst, vm (variation shown in Table 3).
Pectines: Tooth count: 12–12 (♂; Fig. 4A); 11-12 (♀; Fig. 4B) (variation shown in Table 4).
Mesosoma. Tergites I–VI, pre-tergites smooth, post-tergites sparsely, minutely granular, VII minutely granular. Sternites smooth; VII, submedian and lateral carinae weakly developed, smooth to slightly crenulated anteriorly, obsolete posteriorly.
Metasoma. Metasomal segments I–V, dorsal and lateral intercarinal surfaces shagreened to slightly granular; ventral intercarinal surfaces smooth on segments I–III, shagreened on IV–V. Segments I–IV, dorsal lateral carinae weakly to moderately developed (♂) to weakly developed (♀), granular; lateral supramedian carinae moderately developed, granular on I–IV; lateral inframedian carinae weakly developed, granular on I–IV; ventral lateral carinae moderately developed, crenulatedd to smooth on I–III, moderately developed, slightly granular on IV; ventral submedian carinae moderately to strongly developed, crenulated to smooth on I–II, weakly developed, crenulated to granular on III–IV. Segment V length: pedipalp femur length ratio, 1.35 (♂), 1.33 (♀); dorsal lateral carina moderately developed, granular; lateral inframedian carina weakly developed to obsolete, slightly granular; ventral lateral carina strongly developed, granular to slightly serrated; ventral median carina strongly developed, granular to serrated, with large subspiniform granules; ventral transverse carina moderately developed, comprising three subspiniform granules; anal arch semicircular; anal subterminal carina strongly developed, comprising ten subspiniform granules; anal terminal carina moderately developed, granular.
Telson. Telson width: length ratio, 0.42 (♂), or 0.57 (♀). Vesicle, lateral surfaces smooth; ventral surface granular anteriorly, shagreened posteriorly. Subaculear tubercle stout, subconical. Aculeus length 1.3.
Hemispermatophore. Lamelliform, weakly sclerotized (Fig. 7); total length 6.5 mm; distal lamella length 3.4 mm; capsular region, width, 1.2 mm; median lobe narrow, margin entire.
Distribution. D. insularis is only known from the type locality of Cayos Cochinos (specifically Cayo Menor) in north Honduras, and from a nearby island locality of Útila (Fig. 1). Comparable searches were also conducted on the largest island of Roatán, but did not yield any diplocentrids, which may not be present. Cayo Grande was not searched, however given the close proximity and ecological similarity to Cayo Menor, we consider D. insularis to be likely present, although one may note that recently introduced mammals, including feral dogs and cats, appear to have caused some disruption to its ecosystem [23,24] Given the inferred narrow insular geographic range of this new species, we consider it an important priority for conservation assessment (e.g., IUCN red listing), especially on Útila where populations are likely declining as increasing human development is continuing to destroy suitable habitat (Fig. 8).
Ecology. Specimens were collected under rocks and logs, also found under leaf litter at high elevations of the ridge in the island of Cayo Menor, and in the remaining forest patches close to the airport area in the island of Útila, on the eastern side. The main vegetation was evergreen oak forest. Forest floor was covered with high amounts of leaf litter mainly from Quercus and Simarouba trees, and dead wood was frequently encountered, both under which the majority our specimens were collected (Fig. 8). The habitat and habitus of D. insularis n. sp., are consistent with the humicolous ecomorphotype [25].
Biogeography. Útila, Roatán, and other smaller islands form the emergent crest of the continental Bonacca ridge along the Motagua/Swan Islands Fault Zone between the North American and Caribbean plates. Útila is partly formed by basalts from a Quaternary volcano, whilst Roatán and adjacent islands are largely composed of older metamorphic rocks comparable to those in Guatemala, with most recent folding around the Late Eocene, with exhumation and Tela Basin subsidence in the Miocene [26,27]. Cayos Cochinos consists of granite intrusions within metamorphic rocks. Together, the islands appear to be geologically allied to the Sierra de Omoa and/or Cordillera Nombre de Dios mountain ranges on the continental mainland, part of the Middle American Mountain Complex [24,28], with the Islas de la Bahía (Útila, Roatán, etc.) and Cayos Cochinos as topographical high points [24]. The Cayos Cochinos and Útila are situated on the continental shelf, surrounded by shallow 30–55-m-deep waters, and hence connected to mainland during the Pleistocene in the Wisconsin glacial period (ca. 13,000–18,000 years ago) [29]. As the ice sheets melted, rising sea levels would have led Cayos Cochinos and Útila to be increasingly isolated from mainland. We hypothesize that some elements of terrestrial fauna such as diplocentrids could have reached Útila and/or Cayos Cochinos by land connections around this glacial maximum, although uncertainty remains about whether their faunas (such as D. insularis) should then be more closely connected to those of the Sierra de Omoa (i.e. the lineage for D. lourencoi), or to those at the western end (around Tela) of the Cordillera Nombre de Dios (such as D. coddingtoni). If the latter route is more plausible, we might expect closest phylogenetic affinities of D. insularis to D. coddingtoni, and more distinct from others now separated by the Sula valley (i.e. D. lourencoi). In contrast, Roatán and Guanaja are oceanic islands, situated outside the continental shelf and surrounded by deeper waters (up to 275 m in depth) [29]. The drop in sea level of around 120–140 m during the Pleistocene would not have resulted in Roatán and Guanaja, being joined to the mainland, nor to each other, during this period [28,29]. Consequently these older islands therefore appear to have experienced prolonged isolation, and if diplocentrids are later found, we infer they could not have reached those islands via land-bridges.
Given that Cayos Cochinos are now separated from both the mainland and Útila by a relatively shallow sea, other hypothetical modes of marine transport for terrestrial components remain possible, particularly via rafting. For much of the year, easterly trade winds dominate the north coast of Honduras, and eastern surface currents exit from the Gulf of Honduras, with strong flow past Útila towards Cayos Cochinos [30]. This for example might have facilitated later colonization of terrestrial fauna (such as diplocentrids) on Cayos Cochinos from Útila (or other westerly terrestrial sources), particularly through rafting. However, disruption of typical environment occurs relatively frequently in the region though hurricanes, and a counter-clockwise current can also affect the Bay islands early in the year, when plumes of sediment can reach the Cayos Cochinos archipelago from coastal rivers [30]. Consequently, more recent independent colonization of Cayos cochinos by diplocentrids (etc.) appears especially plausible, either rafting from the mainland during environmentally disruptive events (like hurricane damage) or, as we suggest, more likely from Útila via dominant marine currents.
4 Key to the species of genus Diplocentrus in Honduras
| 1. Adult size ranging from 40 to 55 mm. Leg I, telotarsi, counts of spiniform macrosetae in pro- and retroventral rows, 4/4-5. Leg II, basitarsi, spiniform macrosetae pattern, pt, rt, pst, rst, pm, rm, Rm. Pectinal tooth counts, 9–13 (♂) or 9–11 (♀) | 2 |
| –Adult size ranging from 58 to 64 mm. Leg I, telotarsi, counts of spiniform macrosetae in pro- and retroventral rows, 5/5. Leg II, basitarsi, spiniform macrosetae pattern, pt, rt, pst, rst, Rm. Pectinal tooth counts, 13–14 (♂) or 11–13 (♀) | D. santiagoi |
| 2. Legs I–II, telotarsi, counts of spiniform macrosetae in pro- and retroventral rows, 4/4 4/4-5. Leg I, basitarsi, spiniform macrosetae Rm present | D. insularis n. sp. |
| Legs I–II, telotarsi, counts of spiniform macrosetae in pro- and retroventral rows, 4/5 5/5. Leg I, basitarsi, spiniform macrosetae Rm absent | 3 |
| 3. Adult size from 50 to 54 mm. Adult body coloration, dark brown. Pectinal tooth counts, 9–10 (♂) or 8–10 (♀). Pedipalp patella, dorsal retrolateral carina, weakly to moderately developed, smooth to slightly granular (♂) | D. lourencoi |
| –Adult size from 35 to 50 mm. Adult body coloration, brown to reddish brown. Pectinal tooth counts, 11–13 (♂) or 10–11 (♀). Pedipalp patella, dorsal retrolateral carina, obsolete (♂) | D. coddingtoni |
Acknowledgements
The authors thank Operation Wallacea (in particular Dr. Steve Green) for support and funding to initiate what we consider the much-needed terrestrial fieldwork on the arachnids of the Bay Islands. We also thank the support of the Honduras Coral Reef Foundation (HCRF). SJL also thanks Ms. Andrea Martinez of the Kanahau facility for assistance with permits and fieldwork advice on Útila, Miss Tonya and Coral View Beach Resort are thanked to kind hospitality on Útila. Victor Henrriquez is greatly thanked for help and enthusiasm for all aspects of arachnid fieldwork in Honduras. CESL thanks Prof. Oscar Francke for all his support in the recent years. Gerardo Contreras is also thanked for help with photography. Permits were issued through the Instituto Nacional de Conservación y Desarrollo Forestal Áreas Protegidas y Vida Silvestre during 2012/2013 under Dictamen No. DVS-ICF-103-2012 and Resolucion No. DE-MP-118--2012, and 2013/2014 through Dictamen No. DVS-ICF-094-2013 with Resolucion No. DE-MP-088--2013. International export was authorized by the Secretaría de Estado en los Despachos de Agricultura y Ganadería (SAG) associated with No. 17909 and 077-13. The authors thank ICF Departamento de Vida Silvestre Director Lic. Saíd Laínez, Roberto Downing, and Ms. Sindy Clarissa Lagos for help with all aspects of the permitting system.


