1 Introduction
In the last few years, there has been a growing interest in nanomaterial production. Quite recently, considerable attention has been paid to bionanotoxicology due to possible effects and interactions with living organisms [1]. With a size range between 1 and 100 nm, nanoparticles constitute a recently developed technology, largely applied in different fields, such as cosmetics, skin care products, particularly in sun blocks and toothpastes, antibacterial, air cleaning products, and for decomposing organic wastewater treatment, due to their properties, involving high stability as well as anticorrosion and photocatalyst activity [2,3]. For several years, great efforts have been paid to study the types of nanomaterials. Recent researches focus on five groups of NPs, which are carbon nanoparticles, metal oxides, quantum dots, zero value metals, and nanopolymers. Current research on heterogeneous catalysis for catalytic support of a wide variety of metals is focused on titanium dioxide and zinc oxide [4]. Previous studies prove that titanium dioxide nanoparticles (TiO2NPs) are insoluble in water and are thermally stable. They are obtained from minerals such as anatase, rutile, or brookite.
Many researches on the production and use of nanoparticle-based pesticides and compost have been done. Great effort has been devoted to the study of the possible penetration of the nanoparticles into the food chain through plant uptake and their bioaccumulation [5,6]. In the literature, several hypotheses have been proposed to explain phytonanotoxicology. To solve this issue, many researchers have proposed various methods to study plant toxicity. Research on TiO2NP effects on the crops shows a variety of approaches depending on nanoparticles concentration, size, reactivity, chemical structure, surface coating, application, experimental methods, and plant species [7,8].
Previous research has shown that leaf area, stem and root lengths, as well as stem and root weights are morphological indicators of plant health. Antisari et al. [9] argue that the concentrations of 10 and 100 ppm of TiO2NPs at a size of 20 nm enhanced the shoots and roots fresh weights of wheat, whereas concentrations lower than 100 ppm of TiO2NPs reduced them in a dose-dependent manner. Asli and Neumann [10] have shown that 30 and 1000 mg·L−1 of 30-nm-sized TiO2NPs induced leaf growth in Zea mays. However, Fan et al. [11] showed that 35-nm-sized TiO2NPs reduced the number of lateral roots in pea. In addition, Jaberzadeh et al. [12] claimed that TiO2NPs enhanced wheat plant growth and yield.
Besides, on a physiological/biochemical basis, Asli and Neumann [10] proved the negative effect of 30 and 1000 mg·L−1 of TiO2NPs in Zea mays by monitoring the rate of transpiration. Jacob et al. [13] and Servin et al. [14] have demonstrated that TiO2NPs caused a decrease in the chlorophyll content in cucumber and Phaseolus vulgaris leaves. Lei et al. [15] have also found that the TiO2NP treatment caused a decrease in the hydrogen peroxide, superoxide radicals, and malonyldialdehyde contents in the chloroplasts of spinach seedlings due to the activation of superoxide dismutase, catalase, ascorbate peroxidase, and guaiacol peroxidase. Similarly, the results obtained by Jacob et al. [13] suggest that 10 and 30 ppm of TiO2NPs enhanced the antioxidant enzyme activities in Phaseolus vulgaris. In Wang et al. [16], it was shown that 20 μM of TiO2NPs with 2.8 nm caused the excretion of microtubules in Arabidopsis thaliana. As reported by Yang et al. [17] and Mishra et al. [18], TiO2NPs controlled nitrogen metabolism by enhancing enzyme activities and by converting inorganic nitrogen to organic nitrogen into the form of protein and chlorophyll. Another interesting approach to this issue has been proposed by Wang et al. [16], who reported that the effects of TiO2NPs were also on a genetic and molecular basis. Indeed, TiO2NP treatment increased the tubulin monomers that eventually influence the proteosome system in Arabdiopsis thaliana. Castiglione et al. [2] studied the fragmentation of chromosomes in Vicia narbonensis and showed that TiO2NPs caused chromosomal aberrations.
Gao et al. [19] developed a novel sensor using nanoparticles as biostimulants. In this work and in related references, it was observed that TiO2NPs ameliorate light absorbance and conversion from light energy to electrical and chemical energy, and also induced carbon dioxide assimilation by activating rubisco carboxylation, prevented chloroplasts from aging [17,20,21] and induced the expression of rubisco activase genes [22]. Recently, several authors [23–25] have proposed a regulation of photosynthetic rate, water conductance, transpiration rate and growth in plants by TiO2NPs.
However, to the author's knowledge, few publications can be found in the literature that address the issue of the biodisponibility, uptake and transport of TiO2NPs in plants and the mechanism of toxicity on growth and yield. The major drawback of this approach is plant production directly related to the process of photosynthesis. However, most of the previous studies do not consider indirectly the relation of growth and yield with the defense system under TiO2NPs. Siddiqui et al. [7] analyses and compares various aspects of the effects of TiO2NPs in plants. Nevertheless, there are still some interesting and relevant problems to be addressed. Transformation or complexation is a critical factor that affects the fate and toxicity of NPs in living organisms.
Although several studies have indicated differences in the responses of plants to TiO2NPs, little attention has been paid to Fenugreek (Trigonella foenum-graecum L.). It is an annual plant belonging to the family of Fabaceae (Papilionaceae) within the order Leguminosae (Leguminales); it is one of the oldest known medicinal plants. Also, it was cultivated especially in India, in Middle Eastern countries, and largely in the Mediterranean basin, including Tunisia. Because of the presence of important phytochemicals elements such as galactomamnan, disogenin and 4-hydroxyisoleucine, Fenugreek has been proven to be essential for the pharmaceutical, nutraceutical, and functional food industries. It is a phytochemically-rich chemurgic plant with potential agronomic, pharmaceutical, and nutraceutical properties [26]. In addition to its therapeutic effects, it is known to accumulate large quantities of heavy metals [27]. After fenugreek germination, the seedlings produce the first leaf, which is usually simple, since there is still no noticeable epicotyl as the first trifoliate leaf is formed after a further 5–8 days [28]. The paper presents a new approach to examine the impact of TiO2NPs on crop production (development and photosynthesis) and plant protection (tolerance and defense systems). Considering the mentioned impact of TiO2NPs on physiological processes, the hypothesis of this study is that the effects of TiO2NPs on plant production are directly related to the process of photosynthesis or indirectly in relation with the defense system. Growth responses and photosynthetic activities (pigments, total anthocyanins and total soluble proteins) were analyzed. Antioxidative enzyme activities (GPOX, APX and CAT) were determined and membrane peroxidation damage was examined by quantifying MDA production in Fenugreek seedlings exposed to 50 or 100 mg·L−1 of TiO2NPs for 16 days. In this study, a new technique that improves bionanotoxicology is suggested. It provides a new potential research field in applications of NPs.
2 Materials and methods
2.1 TiO2 nanoparticles characteristics
Nano-anatase TiO2 was prepared from a commercial TiO2 nanopowder (Sigma-Aldrich, USA). Titanium (IV) oxide have a purity of 99.7%, their specific surface area is 45–55 m2/g and their formula weight is 79.87 g/mol. X-ray diffraction (XRD) was performed using a Philips X-ray diffractometer with Cu Kα radiation (λ = 0.15406 nm). The crystalline structure of the samples was analyzed using a PAN analytical X’PERT PRO model X-ray diffractometer, on the instrument operating at a voltage of 50 kV and a current of 30 mA. Fourier Transform Infrared spectra were recorded under identical conditions in the 400–4000 cm−1 region using a Fourier Transform Infrared Spectrometer (Shimadzhu). The morphology distribution was characterized using SEM (JEOL JSM 500-F).
2.2 Plant material and growth conditions
Seeds of fenugreek (Trigonella foenum-graecum L.) were purchased from the Space green company, Tunisia. They were disinfected with 2% of sodium hypochlorite for 10 min and rinsed thoroughly to remove the disinfectant and soaked in distilled water at 4 °C for 24 h to obtain an initial stage [29]. The same number of seeds (20 seeds per 9 cm Petri dishes) was germinated in the dark at 24–26°C over two sheets of filter paper imbibed with 10 mL of distilled water for three days. Fenugreek seedlings were then transferred in pots containing Hoagland's nutrient solution [30] containing or not two concentrations of TiO2NPs (50 or 100 mg·L−1). TiO2NP preparations were dispersed by ultrasonic treatment for 60 min and were maintained in the dark. Growth conditions were performed at 25 °C, with relative humidity of 70% and a light–dark cycle of 16:8 h. Nutrient solutions were changed every four days during the experiment. In the greenhouse stage, an experiment was conducted with five replicas, and each pot contained forty seeds.
2.3 Determination of seedlings growth and biomass
The TiO2NP and control seedlings were thoroughly washed with distilled water to remove nanoparticles and nutrients on the surface every four days up to 16 days. The seedlings were then used for growth analyses. Following the harvest of plants, fresh weight (FW) was immediately measured using Electronic Balance and was dried in a hot air oven at 65 °C for 72 h for dry weight (DW) determination. The remaining seedlings were then divided into leaves and stem individually and kept at –80 °C in an ultra-deep freezer for measurements of total soluble protein, pigment contents, antioxidative enzyme activities (GPOX, POX and CAT), and lipid peroxidation (MDA content).
2.4 Quantification of photosynthetic pigments
For the determination of photosynthetic pigments (chlorophyll a, b, and carotenoid levels), 100 mg of fresh leaves from control and treated seedlings were ground with mortar in 5 mL of 80% (v/v) ice-cooled acetone. The pigment extract was measured against a blank of 80% (V/V) acetone at wavelengths of 647 and 663 nm for chlorophyll assays and at wavelengths of 470 nm for carotenoids. The determination of the chlorophyll a, b and carotenoid contents level was carried out using the equations proposed by Lichtenthaler [31] and expressed as mg·g−1 FW.
Chla(μg·mL−1) = 12.21 A663–2.81 A646
Chlb (μg·mL−1) = 20.13 A646 − 5.03 A663
Car (μg·mL−1) = (1000 A470–3.27 Chla–104 Chlb)/229
2.5 Quantification of the anthocyanin content
Anthocyanin samples were analyzed according to Gould et al.’s method [32] Frozen tissues were soaked immediately in acidified methanol (methanol:water:HCl: 16:3:1), then crushed using a glass pestle and kept at 25 °C for 72 hours in the dark. The relative amount of anthocyanin was estimated spectrophotometrically at 530 nm and 653 nm. Determination of anthocyanins level was expressed as μg g−1 FW. Finally, amounts of traits were calculated using the following formula: Anthocyanin (μg·mL−1) = A530–0.24 A653.
2.6 Non-enzymatic antioxidant extraction
After drying at 60 °C in an oven for 72 h, 5 g of powder were introduced into 100 mL of boiling water in a 250-mL Erlenmeyer flask. After 15 min, the sample was filtered, and the filtrate was adjusted to 100 mL with distilled water [33].
2.6.1 Determination of the total flavonoid content
Total flavonoids were determined using the aluminum chloride method reported by Zhishen et al. [34]: 4 mL of H2O and 0.3 mL of NaNO2 (5%) were added to 1 mL of the extract. After 5 min, 0.3 mL of AlCl3 (10%) was added, followed by 2 mL of NaOH (1 M). The final volume was made up to 10 mL with H2O and the solution was mixed. The absorbance was read at 510 nm. Quercetin was used as the standard. The total flavonoid content in the plants was expressed as mg quercetin equivalents (QE)·g−1FW.
2.6.2 Determination of the total phenolic content
Total phenols were determined using the Folin Ciocalteu method reported by Singleton et Rossi [35]. Briefly, 1 mL of Folin Ciocalteu (10%) and 0.8 mL of sodium carbonate (7.5%) were added to 200 μL of the extract. After 30 min, the absorbance was measured at 765 nm. Gallic acid was used as the standard. The total phenolic content in the plants was expressed as μg gallic acid equivalents (GAE)·g−1FW.
2.7 Measurement of the total soluble protein contents
The protein concentration was determined using the BioRad reagent and BSA as a standard [36]. The total soluble protein contents were expressed in mg/FW.
2.8 Estimation of antioxidant enzyme activities
Fresh tissues were ground with a mortar in a homogenization medium (w/v = 1/3) (pH 7.5) consisting of Tris-HCl (50 mmol·L−1), saccharose (0.4 mol·L−1), and EDTA-Na2 (5 mmol·L−1). The homogenate was centrifuged at 800 g for 5 min. The resulting supernatant was centrifuged again at 1500 g for 10 min. The chloroplast pellet was suspended in the homogenization medium and kept at 4 °C.
2.8.1 Guaiacol peroxidase (EC 1.11.1.7) enzyme assay
Guaiacol peroxidase activity was measured following the H2O2-dependent oxidation of guaiacol according to the protocol described by Fielding and Hall [37]. The enzyme extract was added to the reaction mixture containing 50 mM of potassium phosphate (pH 7.0), 10 mM of H2O2, and 9 mM of guaiacol. Enzyme activity was estimated by the increase in absorbance at 470 nm using a UV-Visible spectrophotometer (Lamba 2, PerkinElmer). GPOX activity was determined using the extinction coefficient of 26.6 mM−1·cm−1 and expressed in unit's mg−l protein. One unit of GPOX activity was defined as the amount of enzyme that caused the formation of 1 μM of tetraguaiacol per minute under the assay conditions.
2.8.2 Catalase (CAT, EC 1.11.1.6) enzyme assay
Catalase activity was measured by recording the decomposition of hydrogen peroxide according to the protocol of Aebi [38]. The enzyme extract was added to the reaction mixture containing 50 mM of potassium phosphate buffer (pH 7.0), 10 mM of H2O2, and 1 mM of dithiothreitol (DTT). The enzyme activity was quantified by recording the decrease in absorbance at 240 nm using a UV-Visible spectrophotometer (Lamba 25, PerkinElmer). CAT activity was determined using the extinction coefficient of 39.4 mM−1·cm−1. One unit of CAT activity was defined as the amount of enzyme required to decay 1 mM of hydrogen peroxide/min/mg protein under the assay conditions.
2.8.3 Ascorbate peroxidase (EC 1.11.1.11) enzyme assay
Ascorbate peroxidase activity was determined from the decrease in absorbance at 290 nm (an extinction coefficient of 2.8 mM−1·cm−1) by the method of Nakano and Asada [39]. The reaction mixture contained 50 mM of potassium phosphate (pH 7.0), 0.5 mM of ascorbate, 2 mM of H2O2, 1 mM of EDTA and enzyme extract. Enzyme activity was expressed in terms of l mol of ascorbate oxidized in unit's mg−l of protein.
2.9 Estimation of lipid peroxidation
Lipid peroxidation was estimated by determining the malonyldialdehyde (MDA) [40]. One hundred milligrams of fresh samples were homogenized in 1 mL of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000 g for 10 min at 4 °C. A 0.5-mL aliquot of the supernatant was mixed with 1.5 mL of 0.5% of thiobarbituric acid (TBA) prepared in TCA (20%), and incubated at 90 °C for 20 min. After stopping the reaction in an ice bath, samples were centrifuged at 10 000 g for 5 min. The absorbance of supernatant was then measured at 532 nm. After subtracting the non-specific absorbance at 600 nm, MDA concentration was determined using an extinction coefficient of 155 mM−1·cm−1.
2.10 Statistical analysis
The experimental data were analyzed using two-way ANOVA and differences were considered significant when they reached the 0.05 level of probability.
3 Results and discussion
3.1 XRD, FTIR and SEM
Fig. 1A presents the XRD patterns of the TiO2 powder. The observed XRD peaks are in good agreement with the standard JCPDS file (84–1285), which confirmed an anatase structure at all peaks. Particle size was estimated according to Debye–Scherer's equation [41] as follows (Eq. (1)):
| (1) |
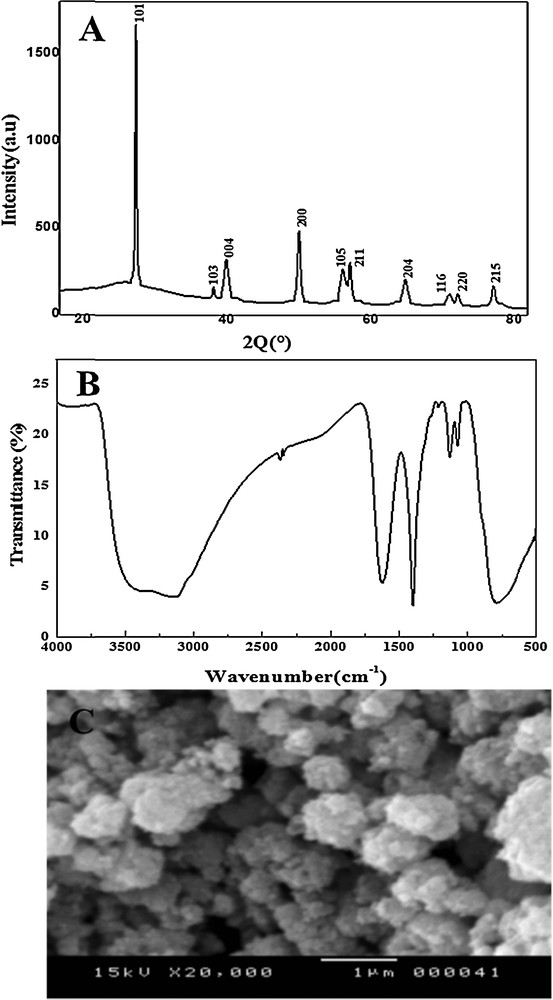
(A) DRX, (B) FTIR, and (C) SEM analyses.
The FT–IR spectrum of anatase TiO2 nanoparticles is shown in Fig. 1B. A broad absorption peak in the range of 3000–3500 cm−1 is present in both figures, which can be attributed to the characteristic absorption of hydroxyl groups (O–H) [42]. The FT–IR spectra of TiO2NPs exhibiting a strong absorption band between 800 and 450 cm−1 remained, which was attributed to the formation of TiO2 nanoparticles.
The surface morphology of the TiO2NPs was studied using SEM. The SEM graph was shown in Fig. 1C for TiO2NPs. It is clear from the SEM of the powder that the size of the particles is less than 20 nm. The SEM results revealed the presence of agglomerates of nanoparticles and showed that the morphology is replete with spheres.
3.2 Effect of TiO2NPs on the growth of fenugreek seedlings
The most important phonological stages are the vegetative growth period, due to its impact on the yields [43]. Various findings on biological impacts of TiO2NPs especially on plant kingdom, have contradictory results, ranging from positive and negative or inconsequential effects [44]. Nanoscale TiO2 proved no significant effects on stem length, stem dry weight (Table 1, Fig. 2). Our results agree with those of Haghighin and Teixeira da Silva [45], who stated that shoot length and shoot dry weight of greenhouse grown seedlings of onion were not significantly affected by TiO2NPs for sizes from 8 to 15 nm. Also, the result corroborates the work, reported earlier, by Seeger et al. [46], who found no differences in the growth of willow trees in the range of 1–1000 mg·L−1TiO2NPs. The same work by Haghighin and Teixeira da Silva [45] proved thatTiO2NPs decreased shoot length in tomato and increased it at most for 100 mg·L−1 of radish, but the dry weight of radish increased in greenhouse. Indeed, Antisari et al. [9] reported that concentrations of 10 and 100 ppm of TiO2NPs with a size of 20 nm enhanced the shoot of wheat. The fresh weight of the stem decreased approximately by 40% over 16 days in 100 mg·L−1 of TiO2NPs. The fresh weight of the stem was reduced by 40% for 100 mg·L−1of TiO2NPs compared to that of controls after 16 days of treatment (Table 1) [9,45]. There was no significant effect on fresh, dry weight of fenugreek leaves of greenhouse grown seedlings between treatments. Internodes increased by 89–100% over 16 days of TiO2NPs treatment, although internodes were not significantly affected by NP-TiO2 treatments compared to control after 16 days of treatment (Table 1).
Fenugreek plant growth after 16 days of treatment with 50 mg/L and 100 mg/L of NP-TiO2 compared to control (H2O).
| Paramètres | NP-TiO2 (mg/L) | Days (d) | |||
| 4 | 8 | 12 | 16 | ||
| Stem length (cm) | 0 | 6.97 ± 0.77 | 6.99 ± 0.51 | 7.2 ± 0.8 | 7.65 ± 0.4 |
| 50 | 7.1 ± 0.75 | 7.41 ± 0.49 | 7 ± 0,9 | 7.53 ± 0.8 | |
| 100 | 6.63 ± 0.56 | 6.85 ± 0.65 | 6.95 ± 0.34 | 7 ± 0.65 | |
| Stem FW (g) | 0 | 0.7 ± 0.04 | 0.67 ± 0.04 | 0.67 ± 0.01 | 0.68 ± 0.07* |
| 50 | 0.63 ± 0.04 | 0.65 ± 0.09 | 0.68 ± 0.06 | 0.59 ± 0.01 | |
| 100 | 0.64 ± 0.02* | 0.65 ± 0.05 | 0.62 ± 0.05 | 0.41 ± 0.01* | |
| Stem DW (g) | 0 | 0.07 ± 0.01 | 0.07 ± 0.005 | 0.07 ± 0.004 | 0.065 ± 0.003 |
| 50 | 0.07 ± 1 × 10−4 | 0.07 ± 0.004 | 0.076 ± 0.003 | 0.06 ± 0.001 | |
| 100 | 0.073 ± 0.03 | 0.075 ± 0.002 | 0.076 ± 0.001 | 0.06 ± 0.003 | |
| Stem H2O (%) | 0 | 89.6 ± 0.96 | 89.1 ± 0.36 | 90.4 ± 1.5 | 86.9 ± 1.3 |
| 50 | 88.3 ± 0.77 | 86.1 ± 0.07 | 88.8 ± 0.6 | 87.7 ± 0.7 | |
| 100 | 88.5 ± 0.77 | 88.5 ± 0.75 | 87.6 ± 0.9 | 84.4 ± 1.7 | |
| Leaves FW (g) | 0 | 0.33 ± 0.03* | 0.47 ± 0.03 | 0.58 ± 0.02 | 0.49 ± 0.1* |
| 50 | 0.27 ± 0.003* | 0.47 ± 0.011 | 0.51 ± 0.104 | 0.54 ± 0.07* | |
| 100 | 0.36 ± 0.04* | 0.38 ± 0.01 | 0.5 ± 0.05 | 0.55 ± 0.04* | |
| Leaves DW (g) | 0 | 0.09 ± 0.003 | 0.085 ± 0.007 | 0.085 ± 0.006 | 0.08 ± 0.01 |
| 50 | 0.095 ± 0.001 | 0.078 ± 0.003 | 0.085 ± 9.4 × 10−5 | 0.082 ± 0.005 | |
| 100 | 0.08 ± 0.003 | 0.088 ± 0.003 | 0.092 ± 0.004 | 0.086 ± 0.002 | |
| Leaves H2O (%) | 0 | 73.9 ± 1.72 | 81.6 ± 1.99 | 84.9 ± 1.86 | 83.1 ± 1.82 |
| 50 | 64.7 ± 0.79 | 83.33 ± 1.03 | 82.7 ± 3.49 | 84.7 ± 1.11 | |
| 100 | 76.3 ± 2.79 | 76.8 ± 1.45 | 81.43 ± 1.11 | 84.2 ± 1.4 | |
| Internodes (cm) | 0 | 0.85 ± 0.21 | 1.01 ± 0.09 | 1 ± 19 × 10−5 | 1 ± 9.5 × 10−5 |
| 50 | 0.49 ± 0.076* | 1.01 ± 1.41 | 1 ± 19 × 10−5 | 1 ± 9.4 × 10−5* | |
| 100 | 0.53 ± 0.04* | 0.89 ± 0.16 | 1.01 ± 0.03 | 1 ± 0.31* | |
| Leaf area (cm) | 0 | 3.57 ± 0.06* | 3.93 ± 0.71 | 4.63 ± 0.24 | 5.03 ± 0.31* |
| 50 | 3.3 ± 0.34* | 3.89 ± 0.3 | 4.99 ± 0.2 | 5.46 ± 0.4* | |
| 100 | 3.23 ± 0.4 | 3.57 ± 0.61 | 4.79 ± 0.35 | 4.04 ± 0.9* |

Effects of 50 mg/L and 100 mg/L of NP-TiO2 on fenugreek (Trigonella foenum-graecum L.) morphology and growth under greenhouse conditions after 16 days of treatment.
It can be concluded that each plant is affected differently by nanoparticle titanium dioxide. Also, research proved that TiO2NPs impacts on the crops depended on nanoparticle concentrations, size, timing, method of application. However, the morphology of nanoparticles also plays an important role in the phytotoxicity. One study reported a significant difference in toxicity to macrophages between two carbon nanotubes with distinct morphologies (MWCNTs and SWCNTs) [47]. Xiang et al. [48] reported that the phytotoxicity of the small ZnO-50 spheres and the large ZnO-90 columns were comparable, which does not support the concept of size-dependent toxicity, instead suggesting a morphology-dependent tendency. The differences in the morphology of nanoparticles may alter the sites and strength of the interaction between the particles and their biological targets, leading to a difference in toxicity.
Toxicity appears to be aggravated by the fibrous or filamentous form of the nanoparticles. Long particles such as nanotubes or nanofilaments would be more toxic than spherical particles of identical chemical composition. This effect is notable for particles with a length greater than 8 μM and a diameter less than 0.25 μM, irrespective of their chemical composition.
3.3 Effect of TiO2NPs on water uptake
Notably, after 16 days of treatment, there were no differences between the water contents of stem and leaves of fenugreek exposed to TiO2NPs compared to controls (Table 1). These results are consistent with the study of Seeger et al. [46], who have shown that TiO2NPs have limited effects on willow tree cutting growth in terms of water usage and transpiration.
3.4 Effect of TiO2NPs on leaf area
Interestingly, at 16 days of stress, higher concentrations of nanoscale TiO2 decreased leaf area by 20% compared to controls (Table 1) and proved a chlorosis (Fig. 3). These results agree with Asli and Neumann [10], who observed that 30 and 1000 mg·L−1 of TiO2NPs with a size of 30 nm inhibited the growth of the leaf of Zea mays.

Symptoms of toxicity of 50 mg/L and 100 mg/L of NP-TiO2 on fenugreek (Trigonella foenum-graecum L.) under greenhouse conditions after 16 days of treatment.
3.5 Effect of TiO2NPs on photosynthetic pigments
Photosynthetic pigments are very important metabolites and play a fundamental role in plant metabolism. The amount of Chl a increased about 16% and threefold due to treatment with 50 and 100 mg·L−1 TiO2NPs respectively after 16 days of treatment. However, the results show that Chl a did not change following the addition of 50 mg·L−1 ofTiO2NPs, but the application of 100 mg·L−1 of TiO2NPs made the Chl a level decrease by 50% compared to controls after 16 days of stress treatment (Fig. 4a), while the amount of Chl b increased by about 145 and 126% under 50 and 100 mg·L−1 TiO2NPs, respectively, for 16 days. The exposure of fenugreek to 50 mg·L−1of NP-TiO2 increased the amount of Chl b by about 36% compared to that of controls after 16 days of treatment. Moreover, 100 mg·L−1 NP-TiO2 significantly diminished by about 51% the amount of Chl b compared to control (Fig. 4b).
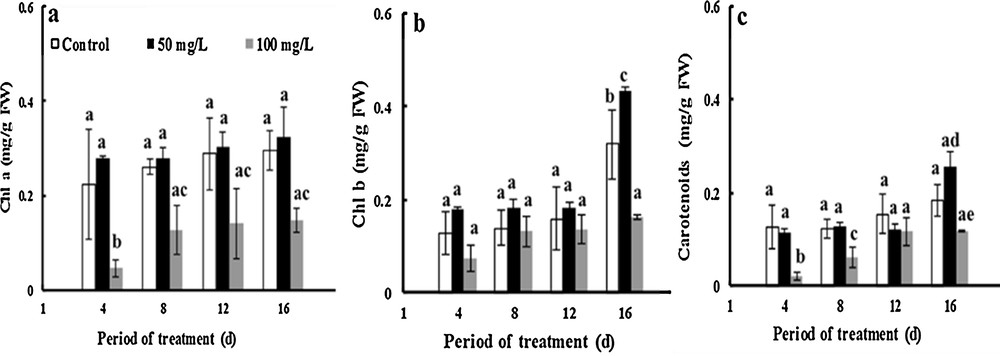
Effects of 50 mg/L (black squares) and 100 mg/L (gray squares) of NP-TiO2 on the photosynthetic pigment (Chl a (a), Chl b (b) and carotenoids (c)) contents in leaves of fenugreek (Trigonella foenum-graecum L.) seedlings compared to control (H2O). Data are means (± SE) of four replicates with one seedlings each. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
In greenhouse experiments, the levels of carotenoids increased by 45% after 16 days of developpement in control plants. The level of carotenoids in TiO2-treatment plant is higherthan that of control. Nanoparticles of TiO2 at concentrations of 50 mg·L−1 increased by 38% the amount of carotenoids compared to the control, although this character declines by 36% after the application of 100 mg·L−1 of TiO2NPs (Fig. 4c).
These results are in accordance with those reported by Lei et al. [24] and Zheng et al. [50]; therefore, Gao et al. [19], in a study on the effects of different concentrations of nanoparticles titanium dioxide on the spinach trait, concluded that the chlorophyll amounts and the photosynthetic rate amount in treatments with NP-TiO2 were significantly increased. The results concur with other studies that have shown that 10 and 30 ppm of TiO2 NPs with size < 25 nm altered chlorophyll contents in Phaseolus vulgaris [13]. Also, Lei et al. [15] observed that 0.25% of NP-TiO2 (colloidal) with a size of 5 nm damaged the chloroplast of the spinach plant, resulting in a reduction of the photosynthetic rate.
These results are in good agreement with others, which have shown that the physiological effects are probably attributed to the small size of the particles, which allows their penetration into the plant during exposure [50,51]. An important implication of these finding is that higher chlorophyll accumulation at a concentration of 50 mg·L−1 TiO2NPs may be due to complementary effects of other inherent nutrients like magnesium (Mg), iron (Fe) and sulfur (S) [52]. TiO2NPs can improve the structure of chlorophyll, increase light absorbance, facilitate the formation of pigments, allow better capture of sunlight and transfer of light energy to active electrons, chemical activities, and have an effect on photosynthesis [52–55]. Leaf chlorosis became pronounced in plants treated with 100 mg·L−1 of TiO2NPs. In agreement with these symptoms and with the reduction in leaf area, significant decreases in Chl a, Chl b and carotenoids were observed under 100 mg·L−1 TiO2 NP stress. The observed decreasing chlorophyll and carotenoids contents may be due to an oxidative process, which is an indicator of tissue ageing, as a result of the stress factors in the environment [56]. At the same time, pigment loss is due to rising levels of toxic metals and metalloids [57]. It can be concluded from this experimental study that the observed chlorosis in leaves and reduction in leaf area were not due to a direct interaction of nanoparticles with the chlorophyll biosynthesis pathway, and that it was caused most probably by a decrease in chloroplast density [33].
Other authors invoked that the decrease in the chlorophyll contents explains that anatase enhances the activity of chlorophyllase [58]. On the other hand, the decreases in Chl a and Chl b at 100 mg·L−1 were due to the damage exerted on the chloroplasts by TiO2. Our results disagreed with those of Cao et al. [59] who stated that no damage was exerted on the chloroplasts by either type of CeO2 NPs at the 100 and 500 mg kg−1 concentrations when increasing chlorophyll a and decreasing chlorophyll b. In our results carotenoids content was less affected by TiO2NPs than chlorophyll. These compounds have antioxidant activity as triplet chlorophyll and singlet oxygen quenchers [60].
3.6 Effect of TiO2NPson anthocyanins content
Under environmental stresses, ROS overproduction can damage important biological molecules (lipids, protein, and DNA) in plants [61]. Plant species have many enzymatic and non-enzymatic antioxidant pathways that can be simultaneously activated to defend against oxidative damage [61,62]. Anthocyanin, a type of flavonoid located in vacuole system [63], is one of most common non-enzymatic antioxidants and operates as a superoxide radical scavenger, hydrogen donor, and metal chelator.
Exposure of fenugreek to TiO2NPs during greenhouse experiments results in an increase in the anthocyanin content: about 2.9- and 1.29-fold for 50 and 100 mg·L−1 of TiO2NPs, respectively, after 16 days of treatment compared to controls (Fig. 5). These results are consistent with those of Kumaret al. [64]; they demonstrated that Pb at concentrations up to 1 mM significantly increased anthocyanin in Ceylon spinach (Talinum triangulare L.). The biosynthesis of anthocyanin in plants is catalyzed by phenylalanine ammonium-lyase (PAL) through a phenylpropanoid pathway [65]. It is possible that highly stressful conditions caused by 100 mg·L−1 NPs may induce the production of high levels of H2O2, which could inhibit the PAL activity, and thus anthocyanin biosynthesis becomes somewhat disrupted.
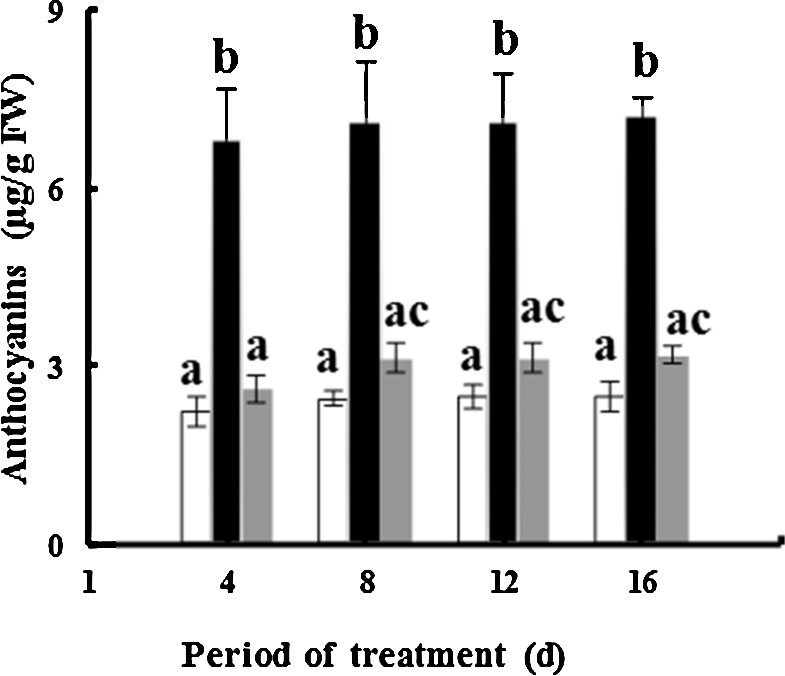
Effects of 50 mg/L (black squares) and 100 mg/L (gray squares) of NP-TiO2 on the anthocyanin contents in leaves of fenugreek (Trigonella foenum-graecum L.) seedlings compared to control (H2O). Data are means (± SE) of four replicates with one seedling each. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
3.7 Effect of TiO2NPs on total phenolics and flavonoids contents
Even after 16 days of treatment, there was a significant decrease in the concentration of flavonoids by about 60 and 78% for 50 and 100 mg·L−1 NP-TiO2, respectively, compared to the control stems. The flavonoid concentration in leaves of fenugreek did not change significantly following the addition of 50 mg·L−1 TiO2NPs, but the flavonoid content was reduced with 100 mg·L−1of TiO2NPs (Fig. 6a). These results disagree with those reported by Rabia et al. [66] in a study on the effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in the micropropagation of Stevia rebaudiana. Bertoni concluded that total flavonoid content was significantly increased by the treatment with NP-TiO2.
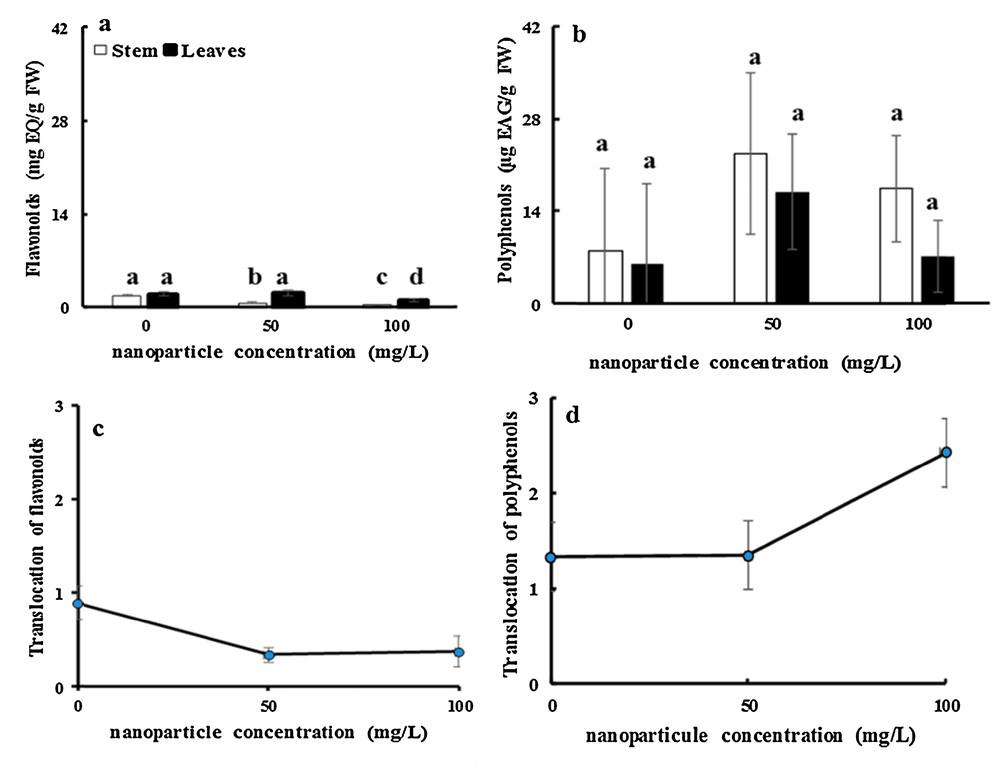
Effects of 50 mg/L and 100 mg/L of NP-TiO2 on flavonoid (a) and polyphenol (b) contents in leaves and stems of fenugreek (Trigonella foenum-graecum L.) seedlings over 16 days compared to control (H2O). Data are means (± SE) of four replicates. The translocation ratios of flavonoids (c) and polyphenols (d) was determined using the contents in stem and leaves. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
Interestingly, after 16 days of stress, the phenol content in stems was improved by factors of approximately 2.8 and 1.45 by a treatment with 50 and 100 mg·L−1TiO2NPs, respectively, compared with the control. The phenol level in leaves enhanced significantly by factors of 2.8 and 1.2 after the application of 50 and 100 mg·L−1TiO2NPs respectively after 16 days of treatment (Fig. 6b).
Fig. 5(c and d) shows that translocation ratio of flavonoids declined significantly by 62 and 71% after treatment with 50 and 100 mg·L−1 TiO2NPs, respectively, after 16 days of treatment (Fig. 6c). Interestingly, in the present experiment, NP-TiO2 stress had no apparent effects on the translocation ratio of polyphenols (Fig. 6d). The decrease in the translocation ratio of flavonoids can be explained by a chelating process. Flavonoids contain an enediol group, by which they act as bidentate ligands for anatase TiO2 nanoparticles. If the chemical bond between flavonoids and TiO2 nanoparticles is strong, desorption of the flavonoids from the anatase surface should be minimal, and thus the replacement of flavonoids with other bidentate ligands present in the vicinity of the nanoparticles (e.g. in a cellular environment) is expected to be negligible. Therefore, anatase TiO2 nanoparticles are predicted to be an efficient platform for the isolation of flavonoids [67].
Transformation or complexation is a critical factor that affects the fate and toxicity of NPs in living organisms. After absorption by roots, plants can transform NPs into other forms [68]. For example, nano-anatase at size 2.8 nm conjugated with alizarin red S accumulate in plantlets of Arabidopsis thaliana grown in hydroponics [69]. These nanoconjugates can pass through cell walls and distribute in distinct subcellular compartments. Peng et al. [70] reported that copper oxide nanoparticles (CuO NPs) entered the stele through lateral roots in rice and translocated to leaves; Cu (II) was mainly combined with cysteine, citrate, and phosphate ligands and was even reduced to Cu (I). For maize, Wang et al. [71] reported that 100 mg·L−1 CuO NPs at size 20–40 nm was translocated from roots to shoots via xylem and re-translocated from shoots to roots via phloem; CuO NPs, during this translocation, could be reduced from Cu (II) to Cu (I). Excess Cu is toxic to plants and caused reduction in growth and yield of rapeseed (Brassica napus L.) and wheat. Parsons et al. [72] observed NiNPs in roots and shoots of mesquite (Prosopis sp.) treated with uncoated nickel hydroxide nanoparticles (Ni(OH)2NPs), whereas leaves had a Ni(II)-organic acid type complex.
3.8 Changes in protein in response to TiO2NP treatment
The data presented in Fig. 6 show that the protein content increased approximately by 24 and 103% in control leaves and stem of fenugreek over 16 days. In leaves, the protein content increased by factors of 1.26 and 4 over 16 days with 50 and 100 mg·L−1 of TiO2NPs. The soluble protein in TiO2NP-treated chloroplast leaves was ∼ 22–64% lower than in controls after 16 days of exposure to 50 and 100 mg·L−1 TiO2NPs, respectively (Fig. 7a). However, in stems of fenugreek plants, the protein levels increased by factors of approximately 1.2 and 3.3 over 16 days with 50 and 100 mg·L−1 TiO2NPs. In addition, there was no impact on the soluble protein content in chloroplast following the exposure to TiO2NPs compared with controls (Fig. 7b). This finding agrees with previous studies by Salama [73], who reported the effects of silver nanoparticles on the protein contents of common bean and corn. The improvement of the protein concentration depends on the optimum dose of nanoparticles of silver for the growth of common bean and corn plants. However, a decrease in the protein contents was observed; it was due to the toxic effect of AgNPs. The soluble protein contents depend also on the content of chlorophyll in the leaves of the plant [74].
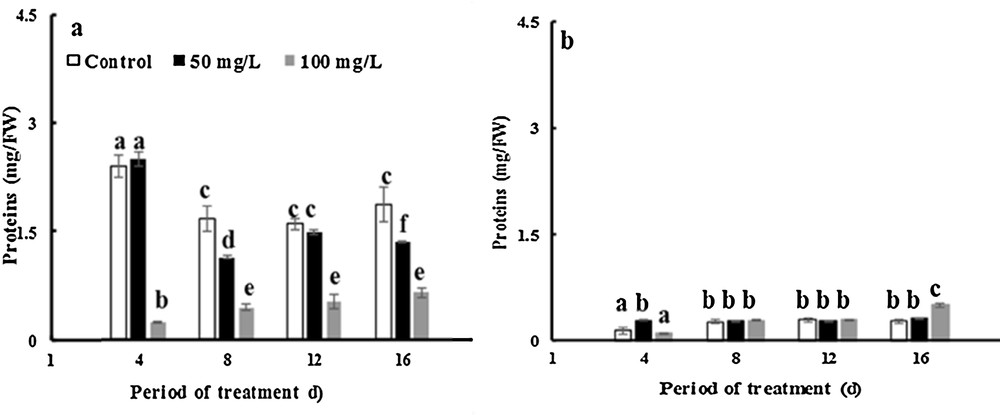
Effects of 50 mg/L (black squares) and 100 mg/L (gray squares) of NP-TiO2 on soluble proteins in the chloroplasts of leaves (a) and stems (b) of fenugreek (Trigonella foenum-graecum L.) seedlings. Data are means (± SE) of four replicates. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
3.9 Effect of TiO2NPs on antioxidants enzymes
The deleterious effects of ROS and lipid peroxidation products are counteracted by the antioxidant defense system [75]. Phenols, flavonoid and stress enzymes are part of the plants defense mechanisms.
GPOX activities increased approximately by a factor of 3 over 16 days in control leaves. They increased by factors of 1.5 and 1.9 compared to controls, due to the treatment with 50 and 100 mg·L−1 of TiO2NPs, respectively, after 16 days (Fig. 8a). CAT activities decreased by approximately 72% during 16 days in control leaves. The activity in TiO2NP-treated leaves was 40–400% higher than in controls after 16 days of exposure for 50 and 100 mg·L−1 of TiO2NPs, respectively (Fig. 8b). APX activity in controls leaves of fenugreek decreased by 21% after 16 days and it was higher than 6.2 and 2.6-fold in TiO2NPs treated leaves compared with normal conditions (Fig. 8c).

Effects of 50 mg/L (square point) and 100 mg/L (Em dash) of NP-TiO2 on GPOX, CAT, and APX activities in the chloroplasts of leaves (a–c) and stems (d–f) of fenugreek (Trigonella foenum-graecum L.) seedlings. Data are means (± SE) of four replicates. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
These results agree with other reports that have shown that nano-anatase treatment could activate SOD, CAT, APX and GPX in spinach chloroplasts. SOD converts O2- into H2O2 and O2; moreover, CAT, APX, and GPX can reduce H2O2 into H2O and O2. Therefore, SOD, CAT, APX, and GPX maintain a low level of ROS, prevent ROS toxicity, and protect cells [15]. GPOX, CAT and APX activities increased approx. 24 and 55% over 16 days in control stems of fenugreek, whereas GPOX and CAT activities did not change significantly following the addition of 50 mg·L−1 of TiO2NPs compared to controls after 16 days of treatment (Fig. 8d–f). Compared to the 100 mg·L−1 TiO2NPs condition, GPOX activity decreased approximately by 11% after 16 days of exposure under control conditions (Fig. 8d). Similar finding noted that CAT and GPOX activities were enhanced in the presence of 10–30 μg·mL−1 of TiO2NPs, but that their activities decreased with higher concentrations of TiO2NPs [76]. Interestingly, in the present experiment, NP-TiO2 stress had no apparent effects on CAT activity (Fig. 8e). No changes in CAT activity in Spirodela polyrrhiza under TiO2 nanoparticles has been previously reported [77]. In contrast, when incubated with NP-TiO2, APX activity was by 95–252% higher than that of controls after 16 days of treatment for 50 and 100 mg·L−1TiO2NPs, respectively (Fig. 8f). Other works reported an increase in CAT activity in response to 250–750 mg kg−1of TiO2NPs, but a decrease in APX activity with 500 mg·kg−1 in cucumber plants [14].
3.10 Effect of TiO2NPs on MDA concentration
Lipid peroxidation measured by the accumulation of the MDA-TBA complex was also assayed in plant tissues (stems and leaves). As shown in Fig. 9, MDA concentration in stems increased significantly by factors of about 1.7 and 2 compared to controls due to the application of 50 and 100 mg·L−1 of TiO2NPs, respectively, after 16 days (Fig. 9a). MDA concentration in NP-TiO2-treated leaves was higher by factors of 1.6 and 1.4 than that of controls after 16 days of exposure for 50 and 100 mg·L−1 TiO2NPs respectively (Fig. 9b). MDA activities significantly increased in nano-stressed leaves, suggesting higher membrane damage. Our results concurred with earlier studies that reported that CuONPs induced an increase in H2O2 and MDA concentrations in the leaves of barley [78], rice, and chickpea seedlings [79,80]. We suggest that fenugreek cells accumulated MDA after decomposition of polyunsaturated fatty acid. Stress induced a progressive enhance in H2O2 content and MDA accumulation indirectly due to oxidative stress. Hydroperoxides were used as markers for oxidative membrane damage [33]. The latter generally acts as an indicator of free radical production [81]. The accumulation of MDA was found to be higher in stems than in leaves. This may be related to the macroscopic signs of toxicity we observed, as membrane damage can induce apoptosis and premature senescence [33].
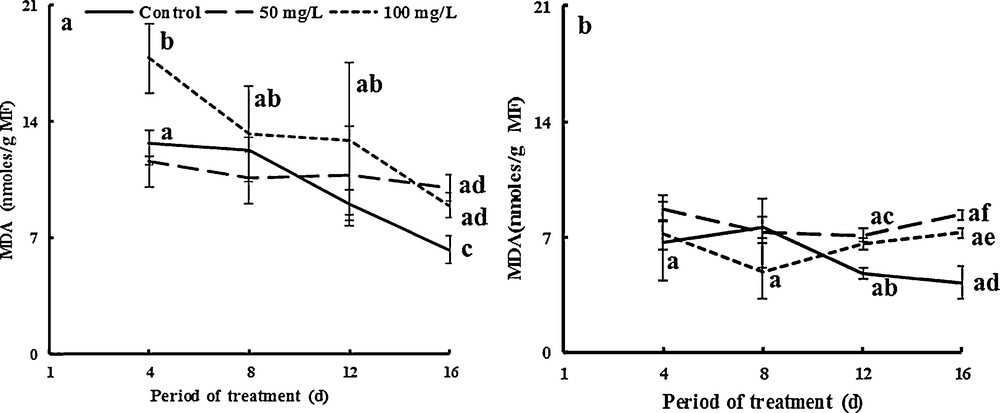
Effects of 50 mg/L (square point) and 100 mg/L (Em dash) of NP-TiO2 on the MDA concentration in leaves (a) and stems (b) of fenugreek (Trigonella foenum-graecum L.) seedlings. Data are means (± SE) of four replicates. Different letters represent significant differences between the treatment means. Differences were considered significant at p < 0.05.
4 Conclusion
Based on the obtained results, it can be concluded that the effects of TiO2NPs on growth and development processes are influenced, not only by the effect on the photosynthetic metabolism, but also by the disturbance of the defense system. Both processes could be considered as indicators of nanoparticle toxicity. The decrease of translocation ratio of flavonoids suggests that they act as bidentate ligands for anatase TiO2NPs. We intend in the future to further investigate the mechanisms of phytotoxicity of NPs, in relation with the size, distribution, and uptake of nanoparticles in plants.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
Financial support for this work was received from the Tunisian Ministry of Higher Education, Scientific Research, and Technology (LR15CERTE04). We are thankful to the anonymous reviewers for helpful comments on the manuscript.


