1 Results and discussion
1.1 Generalities
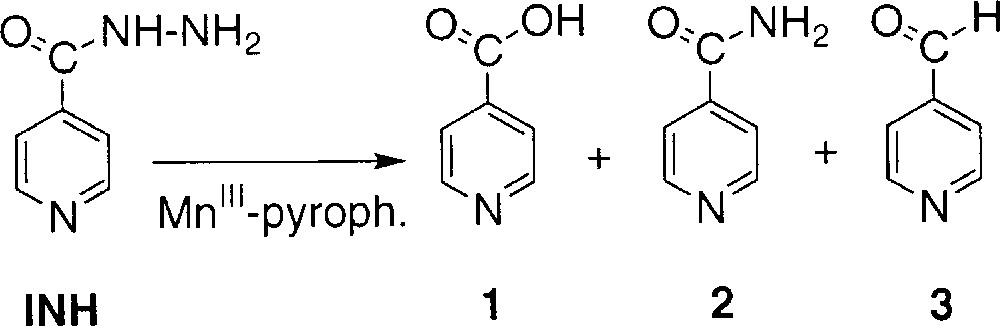
Isoniazid (INH), an antibiotic used in the treatment of tuberculosis 〚1, 2〛, is considered as a prodrug requiring activation by the Mycobacterium tuberculosis KatG enzyme 〚3, 4〛. This catalase–peroxidase hemoprotein 〚4–7〛 catalyses the oxidation of INH but, despite recent efforts, the exact nature of the activated intermediate and the activation mechanism of this drug are still a matter of debate. Since none of the stable derivatives observed in KatG-dependent conversion of INH i.e. isonicotinic acid 1, isonicotinamide 2 and isonicotinaldehyde 3 (Scheme 1) have demonstrated bactericidal effect 〚8〛, the mechanism proposed for INH activity supposes the reaction of a reactive intermediate species with the β-nicotinamide adenine dinucleotide (NAD+/NADH), which is the cofactor of the long-chain 2-trans-enoyl-acyl carrier protein reductase InhA 〚9, 10〛, a key enzyme involved in the biosynthesis of mycolic acids, specific components of the mycobacterial cell wall 〚11, 12〛. The formation of covalent adduct(s) INH-NAD(H) as competitive inhibitor(s) might explain the loss of InhA activity 〚13〛.
Recently, we have shown that a stoichiometric amount of MnIII-pyrophosphate is able to replace the catalysis by the KatG protein in the initial activation of INH and, in the presence of NAD+, to allow the formation of INH-NAD(H) adducts, which are effective inhibitors of the InhA protein 〚14, 15〛. The present work, based on isotopic studies involving D2O, H218O or 18O2 and analyses of INH oxidation products, indicates the simultaneous presence of two reaction pathways, leading to the formation of the isonicotinic acid, with the isonicotinoyl radical as a common intermediate. The isotope content analyses of the amide 2 and the aldehyde 3 are also supporting this mechanism.
Since isonicotinic acid 1 and isonicotinamide 2 do not exchange their carbonyl oxygen with water (Table 1, runs 1 and 2), we could determine the origin of the oxygen atoms in these products and obtain information on their mode of formation. Isonicotinaldehyde 3 exchanged rapidly its carbonyl oxygen atom with water and so is not a convenient reporter for 18O mechanistic study. However, in this case, some data were obtained from reaction performed in D2O. In addition, we checked that neither amide 2 nor aldehyde 3 was transformed in acid 1 by MnIII-pyrophosphate in the experimental conditions described.
Incorporation of 18O () in isonicotinic acid 1 and isonicotinamide 2 during oxidation of INH by MnIII-pyrophosphate (this work) or KatG 〚2〛 (runs 3 and 7) in the presence of H2 or 2. INH does not exchange its oxygen with water. *The pressure was adjusted to 1 atm with nitrogen. The isotopic M+1 contribution (less than 5%) has been included in the M peak.
| Run | Conditions | Oxidation system | Isonicotinic acid 1 | Isonicotinamide 2 | ||||
| M | M + 2 | M + 4 | M | M + 2 | ||||
| CO | C | –C= | CO | C | ||||
| 1 | H2O/air | — | 100% | — | — | 100% | — | |
| 2 | H2/air | — | 100% | — | — | 100% | — | |
| 3 | H2/air | KatG | 33% | 50% | 17% | — | ||
| 4 | H2/Ar (1 atm) | MnIIIpyro. | 9% | 69% | 22% | 100% | — | |
| 5 | H2/air | MnIIIpyro. | 19% | 72% | 9% | 97% | 3% | |
| 6 | H2/O2 (1 atm) | MnIIIpyro. | 35% | 55% | 10% | 97% | 3% | |
| 7 | H2O/2 (1 atm) | KatG | 65% | 35% | — | — | ||
| 8 | H2O/2 (0,2 atm)* | MnIIIpyro. | 80% | 20% | — | 100% | — |
1.2 Formation of isonicotinic acid 1
As shown in Table 1, the incorporation rate of 18O in isonicotinic acid 1 to give M+2 and M+4 species during oxidation of INH by MnIII-pyrophosphate (this work, runs 5 and 8) or by KatG (from ref. 2, runs 3 and 7) is rather similar, suggesting a similar formation pathway with probable common reactive intermediate(s). The level of incorporation of 18O label in 1 differs, in a complementary mode, depending on the use of labelled water (run 5, 81% of labelling) or labelled dioxygen (run 8, 20% of labelling). These results support the existence of two independent routes, the major one (80%) corresponding to the incorporation of oxygen from water and the second one (20%) resulting from the incorporation of oxygen from molecular dioxygen (Scheme 2). In addition:
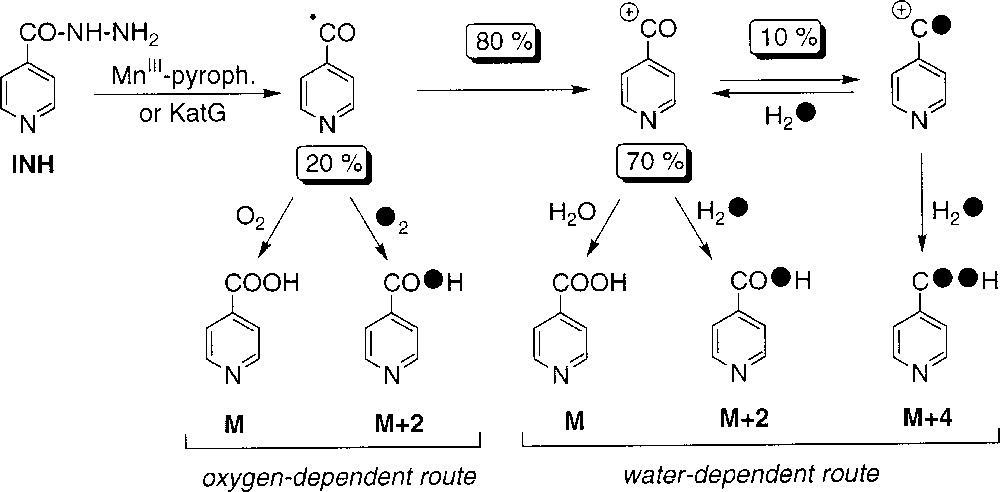
- • (i) in the oxygen-dependent route, the partial incorporation of a maximum of one oxygen atom from dioxygen in compound 1 suggests the formation of an intermediate species, like the isonicotinoyl radical, able to trap dioxygen and giving the acid without exchanging the carbonyl oxygen initially present in INH; this route might involve the formation of a peroxydic radical and its further conversion to 1 catalysed by manganese salts (Scheme 3);
- • (ii) in experiments with H218O, when the partial pressure of dioxygen increases (from about zero, to 0.2 atm and 1 atm, runs 4, 5 and 6, respectively), a concomitant decrease of 18O-incorporation from water (91, 81 and 65%, respectively) was observed – these data suggest that a common intermediate is at the origin of the oxygen- and water-dependent pathways; the difficulty in performing the reaction under an atmosphere completely deprived of dioxygen is illustrated by the 9% value of unlabelled acid 1 observed under argon atmosphere –;
- • (iii) to explain the observed partial double labelling of 1 in experiments performed in H218O (runs 3–6, Table 1), we must suppose the existence of a reaction intermediate allowing the exchange of the carbonyl oxygen atom initially present in INH; this intermediate could be the isonicotinoyl cation generated by oxidation of the isonicotinoyl radical (Scheme 2); the nucleophilic attack of water on this carbocation (Scheme 4) should give the corresponding hydrate which can either loose one proton to give the isonicotinic acid with a single label (pathway 1) or, alternatively, release one water molecule to come back to the carbocation with partial incorporation of a labelled oxygen (pathway 2); finally, the incorporation of a labelled oxygen on this partially labelled cation through addition of another labelled water molecule leads to compound 1 with a partial double 18O labelling; in the usual experimental conditions (run 5), the level of exchange with water of the initial oxygen atom present in INH could be estimated to 10%, based on the amount of detected double-labelled acid 1.
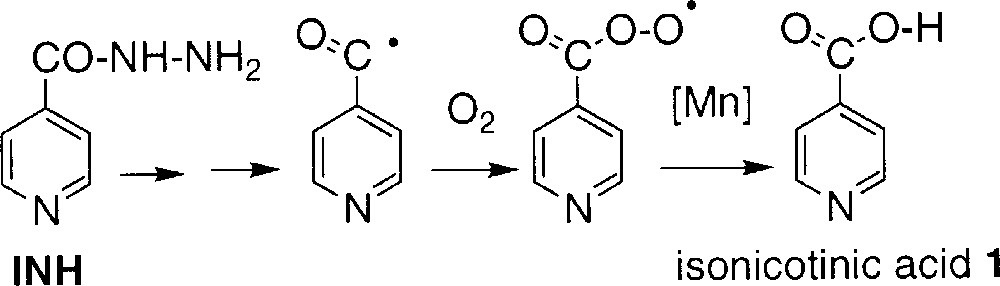
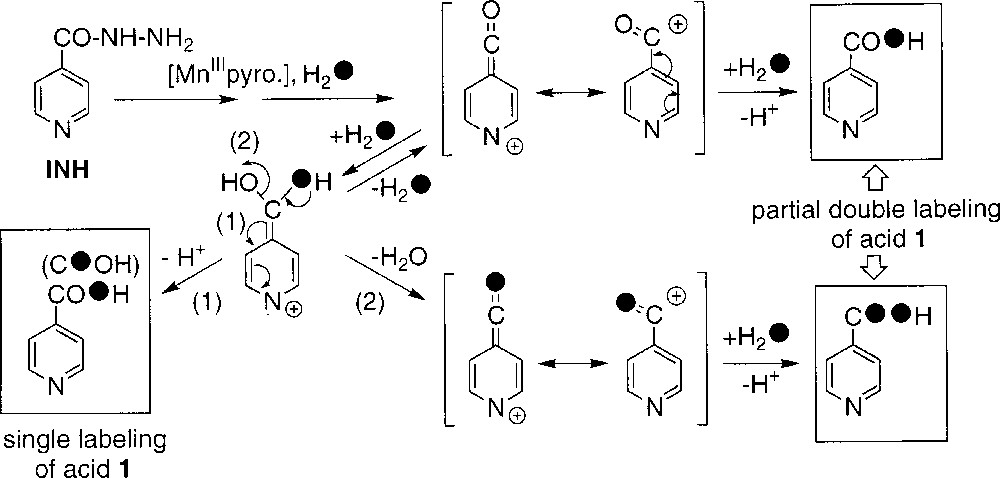
An overall representation of these different ways for generating acid 1 is shown in Scheme 2. Literature data 〚3〛 (runs 3 and 7 in Table 1) suggest that the oxygen-dependent way is slightly more important when the reaction is catalysed by KatG. In addition, both the isonicotinoyl radical and the peroxidic radical could be detected in EPR spin-trapping experiments performed during KatG-mediated oxidation of INH 〚16〛.
1.3 Formation of isonicotinamide 2
Isonicotinamide 2 did not incorporate 18O atom in the presence of H218O or 18O2 (Table 1, runs 2, 4–6, 8). The detection of small levels of 1,2-diisonicotinoylhydrazine in the reaction medium supports a formation pathway with a cleavage of the nitrogen–nitrogen bond, as displayed in Scheme 5 (route a; splitting of the N–N bond to generate amide 2 was also suggested by Schultz 〚3〛) but does not exclude the route proposed by Bodiguel et al., with an initial carbon–nitrogen bond cleavage (route b) 〚17〛.
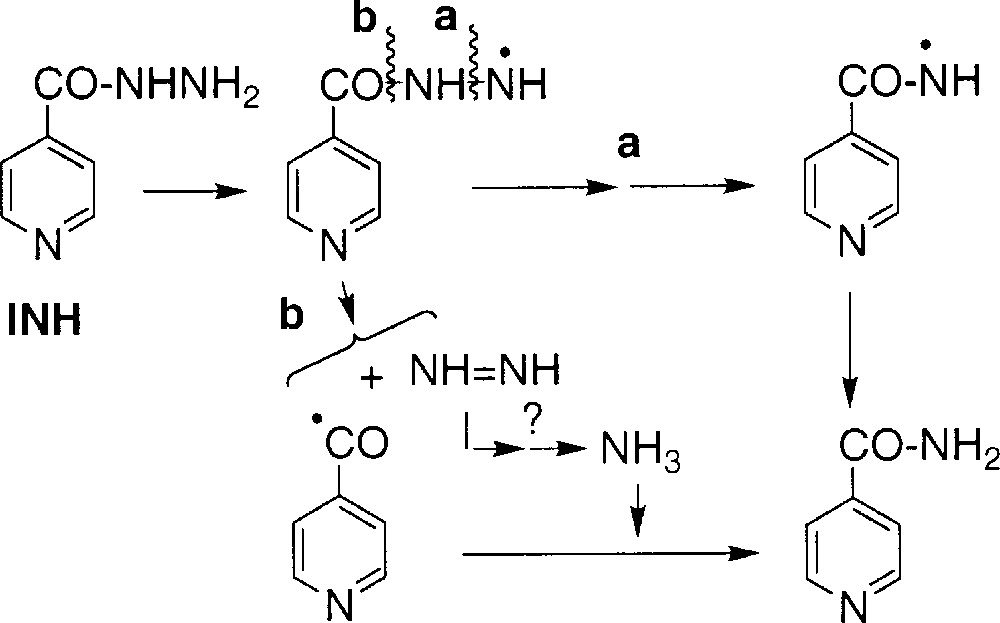
1.4 Experiments in D2O
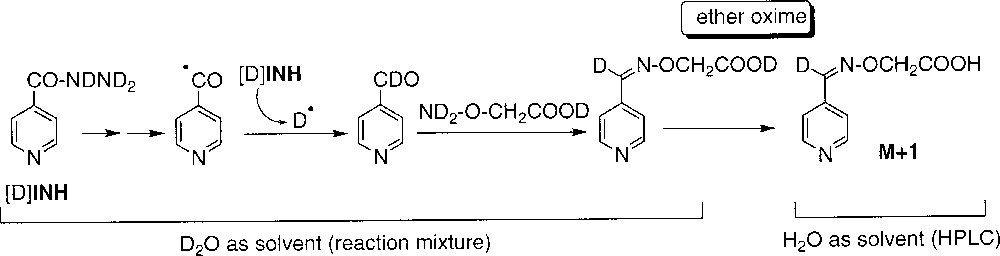
When the reaction was performed in D2O, neither acid 1 nor amide 2 incorporated deuterium. On the opposite, the aldehyde 3 (detected as the O-carboxymethyloxime derivative, since the free aldehyde could not be directly analysed) showed 100% incorporation of deuterium (Table 2, runs 1 and 2). This observation supports the formation of 3 through a radical intermediate, the isonicotinoyl radical, able to abstract D• from D2O or more likely from deuterated INH (fast exchange of the mobile protons of the hydrazide moiety of INH with D2O) (Scheme 6).
Incorporation of deuterium in compounds 1, 2 and 3 during oxidation of INH by MnIII-pyrophosphate with D2O as solvent. *Detected as ether oxime derivative during LC–MS (Scheme 6). All labile deuterium atoms incorporated during the reaction were exchanged for protons during the LC analysis.
| Run | Conditions | Isonicotinic acid 1 | Isonicotinamide 2 | Isonicotinaldéhyde 3* | ||||||
| M | M+1 | M | M+1 | M | M+1 | M+2 | ||||
| 1 | D2O/air | 95% | 5% | 93% | 7% | — | 96% | 4% | ||
| 2 | D2O/N2 | 95% | 5% | 95% | 5% | — | 94% | 6% |
In conclusion, this isotope labelling study provides evidence that the INH oxidation by MnIII-pyrophosphate produces isonicotinic acid 1 through two competitive ways: an oxygen-dependent route and a water-dependent one, both pathways involving the isonicotinoyl radical as a common intermediate. This conclusion is also in agreement with the formation of isonicotinaldehyde 3 through abstraction of an H• from INH by this radical. Since the chemical INH activating system MnIII-pyrophosphate was shown to be a good mimick of the KatG catalase-peroxidase, which activates INH in M. tuberculosis, the isonicotinoyl radical appears likely to be the active species responsible for the activity of INH, and so it should be involved in the formation of the INH-NAD(H) adducts acting as strong inhibitors of key enzymes in the biosynthesis of mycolic acids of bacteria.
2 Experimental section
2.1 Analytical methods
2.1.1 HPLC analyses
Analyses were performed on a reverse-phase C18 column (nucleosil, 10 μm, 250 × 4.6 mm) using a 5:95 (v/v) methanol/NH4OAc 5 mM, pH 4.5 solution as eluent (flow rate: 1 ml min–1). The column was coupled to a diode array detector (Kontron) for the detection of products at 260 nm and the monitoring of UV-Vis spectra. Yields were calculated for INH and compounds 1–3 by comparison with authentic sample calibration curves (in order to detect 3 as its O-carboxymethyl oxime derivative, some reaction samples were analysed after 5 min quenching with 40 mM NH2OCH2COOH).
2.1.2 LC–TIS–MS analyses
The analyses were performed in the conditions indicated above, but with a quaternary pump. Only 50% (split 1/2) of the flow eluted from the column was introduced into the electrospray turbo ionisation source (TIS). Nitrogen at 480 °C (ultrahigh purity) was used as spray and drying gas. The ESI–MS spectrometer was a Perkin-Elmer SCIEX API 365 and the analyses were performed in the positive mode.
2.2 Materials
Manganese(III)-pyrophosphate, MnIII(H2P2O7)3〛Na3, was prepared according to previously described method 〚14, 18〛. Isoniazid, isonicotinic acid, isonicotinamide, and isonicotinaldehyde were obtained from Sigma-Aldrich. H218O (97.1 atom% ) was supplied by Eurisotop, D2O (> 99.9 atom% ) by SDS (Peypin, France) and 18O2 (96.8 atom% ) by Leman (Saint-Quentin-en-Yvelines, France).
2.3 Oxidation conditions
The reaction medium (final volume of 1 ml of water for classical HPLC analysis or 100 μl for LC–TIS–MS analysis), containing 50 mM phosphate buffer pH 7.5, 0.5 mM INH and 1 mM MnIII-pyrophosphate (introduced in five consecutive additions of 200 μM each, every 2 min) was stirred at room temperature for 10 min and then analysed by HPLC or LC–TIS–MS after 5 min quenching with 40 mM NH2OCH2COOH (200 μM 2-nitrobenzoic acid was used as internal standard only for HPLC analysis). In a typical reaction, the yields of compounds 1, 2 and 3 were 45, 12 and 4% , respectively.
2.4 Experiments with H218O
Solutions of required concentrations and volume of isoniazid (0.5 mM) plus phosphate buffer (50 mM, pH 7.5), MnIII-pyrophosphate (1 mM) and NH2OCH2COOH (40 mM) were prepared separately and lyophilised using a Speed-Vac. Appropriate volumes of H218O were added (50 μl for isoniazid in buffer sample, 40 μl for MnIII-pyrophosphate sample and 10 μl for NH2OCH2COOH sample; the total volume was 100 μl) and the reaction was carried out as described above. In case of reaction under dioxygen or argon, the reaction medium was placed in a Schlenk tube connected to a pressure detector and degassed by three consecutive freeze-thaw cycles under vacuum. It was then repressurised with 1 atm of dioxygen or argon. The solution of MnIII-pyrophosphate (previously degassed) was added in five consecutive additions via a gastight syringe. After 10 min, the reaction medium was quenched with a previously degassed solution of NH2OCH2COOH. After 15 min, the sample resulting from isoniazid oxidation was directly analysed by LC–TIS–MS. The percentage of 18O incorporated in each product was determined by the relative abundances of the peaks (peak surfaces) at m/z 124, 126 or 128 (M+H+ of isonicotinic acid 1 with no, one or two 18O atom incorporated) and m/z 123 or 125 (M+H+ of isonicotinamide 2 with no or one 18O atom incorporated). The values obtained were corrected considering the 18O content of labelled water. We also checked (except for isonicotinaldehyde, which was derivatised with NH2OCH2COOH in ether oxime) that there was no oxygen exchange with H218O of isoniazid or its oxidation products 1 and 2.
2.5 Experiments with D2O
The conditions were the same as for experiments with H218O, but in this case appropriate volumes of D2O were added. The incorporation of deuterium in each product was followed by the relative abundances of the peaks (peak surfaces) at m/z 124 and 125 (M+H+ of isonicotinic acid 1 with no and one deuterium incorporated) and m/z 123 and 124 (M+H+ of isonicotinamide 2 with no and one deuterium incorporated).
2.6 Experiments with 18O2
The conditions were the same as for experiments with H218O under dioxygen or argon but, in this case, after three consecutive freeze-thaw cycles, the reaction medium was repressurised with the desired pressure of 18O2 (0.2 atm) and nitrogen was then added to have a final pressure of 1 atm. After 15 min, the sample was analysed by LC–TIS–MS. The values obtained for 18O incorporations were corrected considering the 18O content of the labelled dioxygen.
Acknowledgements
All ESI-MS analyses have been performed in the ‘Service de spectrométrie de masse FR14–LCC’ (Toulouse, France), with the collaboration of Suzy Richelme.



