1 Introduction
Addition of Grignard reagents onto esters is well known to deliver tertiary alcohols and is therefore of limited synthetic interest. When the same reaction is performed in the presence of reductive agents like hydrides, competition can occur between the two nucleophilic species to furnish selectively secondary alcohols 〚1–4〛. For example, a combination of RMgX/zinc borohydride 〚5〛 in THF has been recently reported to allow, in one single step, an easy access to this class of alcohols 〚6〛. In the case of vinyl magnesium bromide, the reaction led to substituted 4-penten-1-ol derivatives, resulting unambiguously from a second addition of the Grignard reagent onto a conjugated enone intermediate. Furthermore, the adducts could be subsequently transformed into tetrahydrofuran derivatives 〚7〛 (Fig. 1).
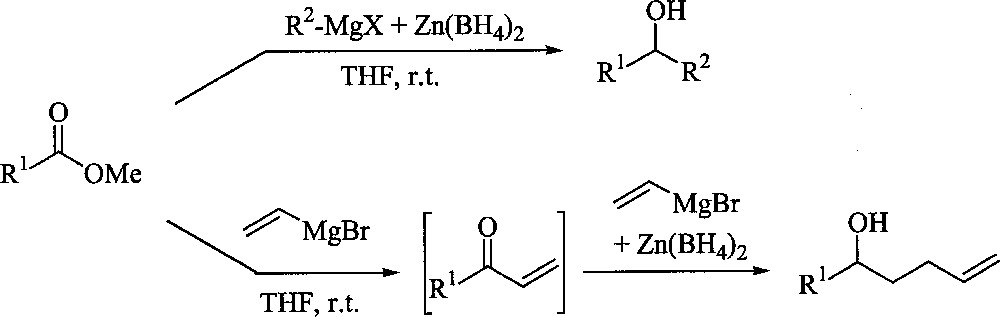
Reductive alkylation of esters.
Numerous natural products (e.g., insect pheromones) possess a lactone framework 1 〚8, 9〛 and especially a butyrolactone subunit (n = 0). As part of our interest into tandem and one-pot procedures 〚10〛, we wish to present therein a new and direct access to this important type of compounds from commercially available anhydrides 2 or unsubstituted lactones 3.
2 Results
The two sequences of our investigation are summarised in Fig. 2.
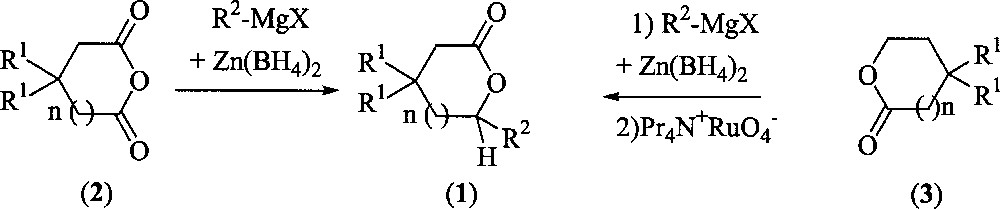
Synthesis of substituted lactones from anhydrides or from unsubstituted lactones.
2.1 Synthesis of lactones from anhydrides
We first submitted anhydrides 2 to the action of different Grignard agents combined with Zn(BH4)2, in order to obtain hydroxy acids. After hydrolysis and extraction, the resulting crude mixtures were directly heated to promote lactonisation by simple azeotropic removal of water under moderate acidic conditions (Fig. 3). Results are collected in Table 1.
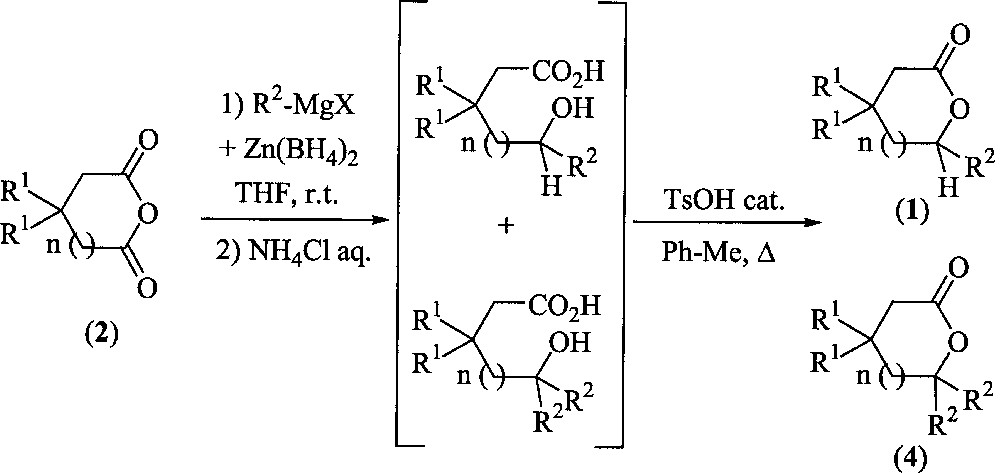
Synthesis of lactones from anhydrides.
Reductive alkylation of anhydrides 2.
| 2 | R1, R1 | n | R2–MgX | 1 | 4 |
| 2a | H, H | 0 | Ph–MgCl a | 1a (44%) | 4a (11%) |
| 2a | H,H | 0 | Ph–MgCl b | 1a (45%) | 4a (10%) |
| 2a | H, H | 0 | PhCH2–MgCl b | 1b (16%) | 4b (3%) |
| 2a | H, H | 0 | n-C8H17–MgCl b | 1c (28%) | — |
| 2b | H, H | 1 | PhCH2–MgCl b | 1d (15%) | — |
| 2c | –(CH2)4– | 1 | PhCH2–MgCl b | 1e (33%) | — |
The molecular ratio of anhydride /R–MgX/Zn(BH4)2 to use was first determined on anhydride 2a (Table 1, entries 1 and 2). No significant change was observed by using 1:4:0.25 or 1:2:0.25. Therefore, experiments on other substrates were conducted with the second ratio. Compounds 1a–e and 4a–b were conveniently isolated by flash-chromatography on silica and easily characterised according to their spectroscopic properties and/or by comparison with literature data (see experimental part). Compared to the reaction performed on esters 〚6〛, the overall yield and the selectivity of mono- and dialkylated products are lower (1/4 = 4:1). Due to their ring strain, anhydrides could react more rapidly 〚11〛 with the less hindered nucleophilic species (hydrides versus Grignard reagents) to deliver highly hydrophilic and/or volatile compounds, difficult to isolate.
2.2 Synthesis of lactones from unsubstituted lactones
In order to improve the chemical yields of monosubstituted lactones (1), we next decided to examine a two step procedure by submitting lactones (3a–c) to the same reductive alkylation conditions (molecular ratio: (3)/R–MgX/Zn(BH4)2 = 1:2:0.25). In this case, the resulting diols 5 or 6 can be further converted into compounds 1 or 4 by a selective oxidation of the primary alcohol. Results are summarised in Fig. 4 and Table 2.
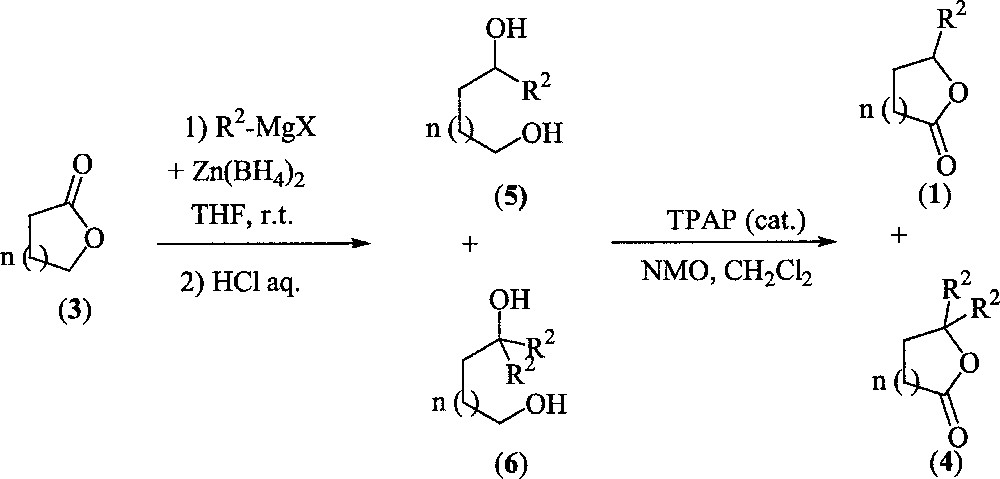
Alkylation of lactones.
Two-step preparation of monosubstituted lactones 1.
| 3 | n | R2–MgX | 5 | 6 | 1 b |
| 3a | 1 | PhCH2–MgCl | 5a (20%) | 6a (10%) | 1b (80%) |
| 3a | 1 | C8H17–MgCl | 5b (43%) | 6b (19%) | 1c (90%) |
| 3b | 2 | C11H23–MgBr | 5c (20%) | — | 1f (30%) |
| 3c | 3 | C11H23–MgBr | 5d (22%) | — | — a |
According to the third experiment, compound 5c was isolated in only 20%. The main side-product was isolated in 66% and was identified as 1,5-pentanediol, corresponding to the double reduction with hydrides of the carboxylic group. This observation indicates unambiguously that reduction occurred more rapidly than addition of the Grignard reagent. Finally, the oxidation step promoted by catalytic amounts of TPAP 〚12〛 afforded lactones 1b and 1c in excellent yields, respectively from 5a and 5b. With diol 5d (n = 3), oxidation led to the formation of a complex mixture of esters instead of the expected seven-member ring lactone. This clearly results from a two-step sequence: oxidation of the primary alcohol function of 5d led to the corresponding aldehyde, which was trapped with an additional molecule to give an intermediate lactol, oxidised in situ into an ester.
3 Conclusion
In conclusion, the combination of a Grignard reagent and zinc borohydride has been used for the synthesis of monosubstituted lactones, starting from commercially available anhydrides or butyro- and valerolactones. By this mean, 4-dodecanolide (1c) and 5-hexadecanolide (1f) have been prepared, which are respectively pheromone of rove beetle (Bredius mandibularis) and of the queens of orientalis hornet (Vespa orientalis) 〚9〛. The overall yields and the selectivities are quite low as compared to results observed with esters under similar conditions; the competitive double reduction of the carboxylic functionality has been attributed to the ring strain of the substrates. However, due to the simplicity and to the availability of the starting materials, these conditions could be of interest to prepare related monosubstituted lactones and are competitive with other syntheses already published for that type of compounds.
4 Experimental part
NMR spectra were recorded using a Bruker ALS 300 (300 M Hz for 1H NMR, 75 M Hz for 13C NMR) instrument. All the samples were diluted in CDCl3. IR spectra were measured with a Perkin-Elmer Spectrum One apparatus. Anhydrous solvents have been distilled on sodium–benzophenone. Given yields are all isolated yields.
4.1 General procedure for the synthesis of lactones starting from anhydride
A solution of Zn(BH4)2 in THF (2.5 mmol) was added under N2 to the Grignard reagent (20 mmol) already prepared in the same solvent. After 1 h at room temperature, a solution of anhydride 2 (10 mmol) in THF (2 ml) was dropwise added. After 45 min, the resulting mixture was carefully hydrolysed with a saturated solution of ammonium chloride (10 ml). After extraction with ether (3 × 25 ml), the organic layers were dried over MgSO4 and concentrated under reduced pressure. The crude product was next diluted into toluene (40 ml) and heated in the presence of catalytic amounts of TsOH for 4 h, using a Dean-Stark apparatus. The solvent was distilled off and the product 1 was isolated after flash-chromatography on silica (eluent: petroleum ether (PE)/AcOEt 80:20).
4.2 General procedure for the synthesis of lactones starting from lactone
A solution of Zn(BH4)2 in THF (2.5 mmol) was added under N2 to the Grignard reagent (20 mmol) already prepared in the same solvent. After 1 h at room temperature, a solution of lactone 3 (10 mmol) in THF (5 ml) was dropwise added. After 45 min, the resulting mixture was carefully hydrolysed with a saturated solution of ammonium chloride (10 ml). After extraction with ethyl acetate (3 × 25 ml), the organic layers were dried over MgSO4 and the solvent removed by distillation. Diol 5 was isolated after flash-chromatography on silica (eluent: PE/AcOEt 80:20). 5 was next dissolved into CH2Cl2 (30 ml) containing 3-Å molecular sieves, TPAP (1 mmol) and N-methylmorpholine oxide (3 mmol) were added at 0 °C. After 20 h, the crude mixture was filtered off and the solvent removed. Lactone 1 was isolated from the crude mixture by flash-chromatography on silica (eluent: PE/AcOEt 80:20).
4.3 5-Phenyl-dihydro-furan-2-one (1a) 〚13〛
1H NMR δ 2.21 (m, 1H); 2.66 (m, 3H); 5.52 (dd, 1H, J = 6.6 Hz; J = 7.35 Hz); 7.35 (m, 5H); 13C NMR 29.0; 31.0; 81.2; 125.3; 128.5; 128.8; 139.4; 177.0; IR (cm–1): 3035; 2924; 1772; 1497; 1455, 1175; 1141; 1021; 939; 699.
4.4 5-Benzyl-dihydro-furan-2-one (1b) 〚14〛
1H NMR: δ 1.91 (m, 1H), 1.99 (m, 1H), 2.4 (m, 2H), 2.93 (dd, 1H, J = 14 Hz; J = 5.9 Hz), 3.07 (dd, 1H, J = 14 Hz; J = 6.6 Hz), 4.74(m, 1H, J = 5.9 Hz, J = 6.6 Hz), 7.24 (m, 5H); 13C NMR 27, 29, 41, 81, 127, 128.7, 129.5, 130 (Car); 177 (C = O); IR (cm–1): 2927, 1772, 1498, 1455, 1354, 1177, 1022, 921, 750, 702.
4.5 5-Octyl-dihydro-furan-2-one (1c) 〚15〛
1H NMR: δ 0.89 (m, 3H); 1.28–1.66 (m, 12H), 1.74–1.88 (m, 2H), 2.33 (m, 2H), 2.55 (dd, 2H, J = 9.63 Hz, J = 6.78 Hz), 4.5 (m, 1H); 13C NMR 14.1, 22.7, 25.2, 28.0, 28.9, 29.2, 29.4, 29.5, 31.9, 35.6, 81.1, 177.3; IR (cm–1): 2927, 2856, 1777, 1460, 1180, 1016, 980, 914.
4.6 6-Benzyl-tetrahydro-pyran-2-one (1d) 〚16〛
1H NMR: δ 1.56 (m, 2H), 1.83 (m, 2H), 2.4 (t, 2H, J = 7.3 Hz); 2.75 (dd, 1H, J = 13.2 Hz, J = 4.4 Hz), 2.81 (dd, 1H, J = 13.2 Hz, J = 8.1 Hz), 3.8 (m, 1H), 7.3 (m, 5H); 13C NMR 18.4, 27.1, 29.5, 42.1, 72, 81.0, 127.0, 128.6, 129.6, 136, 171.6; IR (cm–1): 3063, 3029, 2927, 2871, 1732, 1454, 1245, 1175, 1082, 1042, 746, 699.
4.7 9-Benzyl-8-oxa-spiro〚4,5〛decan-7-one (1e)
1H NMR: δ 1.58 (m, 10H), 2.23 (1H, d, J = 16.8 Hz), 2.49 (1H, d, J = 16.8 Hz), 2.89 (1H, dd, J = 13.8, J = 6.6 Hz), 3.09 (1H, dd, J = 13.8, J = 6.0 Hz), 4.58 (1H, m), 7.21–7.42 (5H, m); 13C NMR: 23.6, 24.0, 37.3, 39.9, 40.7, 40.8, 42.1, 42.3, 78.9, 126.8, 128.6, 129.6, 136.5, 171.7; IR (cm–1) 3062, 3028, 2925, 1733, 1450, 1082; MS (m/z, relative intensity): 244 (M+, 8), 153 (100), 109 (30), 81 (23).
4.8 6-Undecyl-tetrahydro-pyran-2-one (1f) 〚17〛
1H NMR: δ 0.9 (m, 3H), 1.28 (m, 18H), 1.57 (m, 2H), 1.9 (m, 4H), 2.5 (m, 2H), 4.3 (m, 1H); 13C NMR 14.1, 18.5, 22.7, 23.8, 25.0, 27.8, 29.3, 29.4, 29.5, 29.6, 29.7, 31.9, 35.9, 80.6, 172.0; IR (cm–1): 2925, 2854, 1734, 1465, 1243, 1172, 1049.
4.9 5,5 Diphenyl-dihydro-furan-2-one (4a) 〚18〛
1H NMR: δ 2.59 (t, 2H, J = 8.1 Hz), 2.92 (t, 2H, J = 8.1 Hz), 7.23 (m, 10H); 13C NMR 29.1, 35.7, 89.8, 125.4, 127.9, 128.6, 143.1, 176.1; IR (cm–1): 3061, 2924, 1778, 1598, 1493, 1449, 1220, 1164, 1044, 981, 921, 755.
4.10 5,5-Dibenzyl-dihydro-furan-2-one (4b) 〚19〛
1H NMR: δ 1.7 (t, 2H, J = 8.8 Hz), 2.08 (t, 2H, J = 8.8 Hz), 2.88 (d, 2H, J = 14 Hz), 3.12 (d, 2H, J = 14 Hz), 7.28 (m, 10H); 13C NMR 28.8, 29.2, 46.3, 87.7, 127.1, 128.5, 130.7, 135.5, 176.9; IR (cm–1): 3029, 2919, 1772, 1495, 1455, 1179, 1082, 1027, 933, 702.
4.11 5-Phenyl-pentane-1,4-diol (5a) 〚20〛
1H NMR: δ 1.75 (m, 4H), 2.72 (dd, 1H, J = 8.2 Hz, J = 13.5 Hz), 2.85 (dd, 1H, J = 4.6 Hz, J = 13.5 Hz), 3.71 (m, 2H), 3.9 (m, 1H), 7.3 (m, 5H).
4.12 Dodecane-1,4-diol (5b) 〚21〛
1H NMR: δ 0.88 (m, 3H), 1.25 (m, 12H), 1.50 (m, 2H), 1.75 (m, 4H), 2.18 (s, 1H), 3.6 (m, 3H); IR (cm–1): 3334, 2926, 2855, 1466, 1056.
4.13 Hexadecane-1,5-diol (5c) 〚22〛
1H NMR: δ 0.82 (t, J = 7 Hz, 3H), 1.18 (m, 20H), 1.3 (m, 4H), 1.5 (m, 2H), 3.6 (m, 3H); 13C NMR 14.1, 21.9, 22.7, 25.7, 29.3, 29.6, 31.9, 32.6, 37.0, 37.6, 62.8, 71.9; IR (cm–1): 3254, 2917, 2849, 1466.
4.14 Heptadecane-1,6-diol (5d) 〚23〛
1H NMR: δ 0.89 (t, 3H, J = 6.7 Hz), 1.27 (m, 22H), 1.53 (m, 4H), 1.68 (m, 2H), 2.11 (s, 2H), 3.6 (m, 3H); 13C NMR: 10.0, 14.1, 25.3, 25.8, 29.4, 29.7, 30.3, 30.5, 30.9, 31.9, 32.8, 33.3, 33.5, 34, 37.8, 72.1, 73.5; IR(cm–1): 3254, 2956, 2917, 2849, 1467.
4.15 4-Benzyl-5-phenyl-pentane-1,4-diol (6a) 〚19〛
1H NMR: δ 1.81 (m, 4H), 2.84 (s, 4H), 3.6 (t, 2H, J = 6.2 Hz), 7.3 (m, 10H); IR (cm–1): 3390; 3061; 3028; 2946; 2874; 1602; 1582; 1495; 1454; 1338; 1183; 1090; 1053; 1030; 1005; 910; 884; 790; 753; 701; 635.
4.16 4-Octyl-dodecane-1,4-diol (6b) 〚24〛
1H NMR: δ 0.85 (m, 6H), 1.25 (m, 24H), 1.4(m, 2H), 1.6 (m, 1H), 1.95 (s, 1H), 3.65 (t, 2H, J = 5.7 Hz); 13C NMR: 14.5, 23.1, 24.0, 27.0, 29.7, 30.0, 30.7, 32.3, 36.5, 39.5, 63.6, 74.7.
Acknowledgements
O.P. acknowledges the French CNRS for its financial support (AIP ‘Jeune Équipe’, 1999–2001).


