We have recently described the efficient chemical synthesis of the 6A,6D-butylene-bridged α-cyclodextrin (CD) 3α. As shown in Fig. 1 [1], this capped α-CD 3α was easily derived from 1α, a diol directly obtained in high yield through a diisobutylaluminium (DIBAL)-promoted regioselective de-O-benzylation of perbenzylated α-CD.
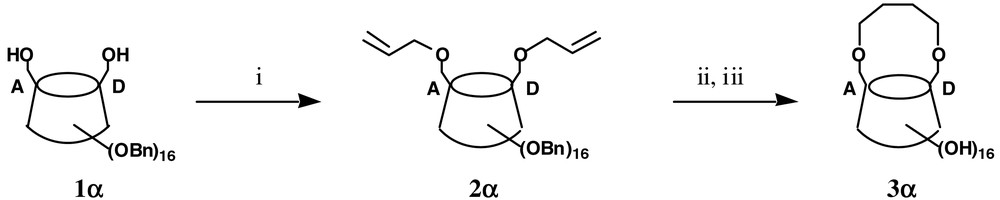
(i) NaH, AllylBr, DMF, rt (92%); (ii) Cl2 (PCy3)2 Ru=CHPh (6 mol%), PhH, 60 °C; (iii) H2, Pd/C 10%, Pd black, EtOAc/MeOH (1:1), 48 h, rt (87% over two steps).
In this preliminary communication, we would like to report on the extension of this reaction to the synthesis of a new family of 6A,6D-capped cyclodextrins, together with a preliminary evaluation of their inclusion properties.
The corresponding 6A,6D-butylene-capped β-CD 3β was first prepared in a similar manner (Fig. 2) from the known diol 1β [1]. It is worth noting that 3β has also been very recently synthesised using this procedure [2]. The per-O-methylated derivative was able to separate enantiomers of various molecules and shows high selectivity towards large and voluminous molecules. Selected data for 3β: [α]D20 = +129 (c = 0.2, MeOH); MS (MALDI-TOF): m/z (%): 1211.5 (100) [M + Na+]; 13C NMR (100 MHz, D2O): 102.2, 102.1, 101.6, 101.4, 101.2, 100.4, 99.5 (7 × C1), 26.2, 25.8 .
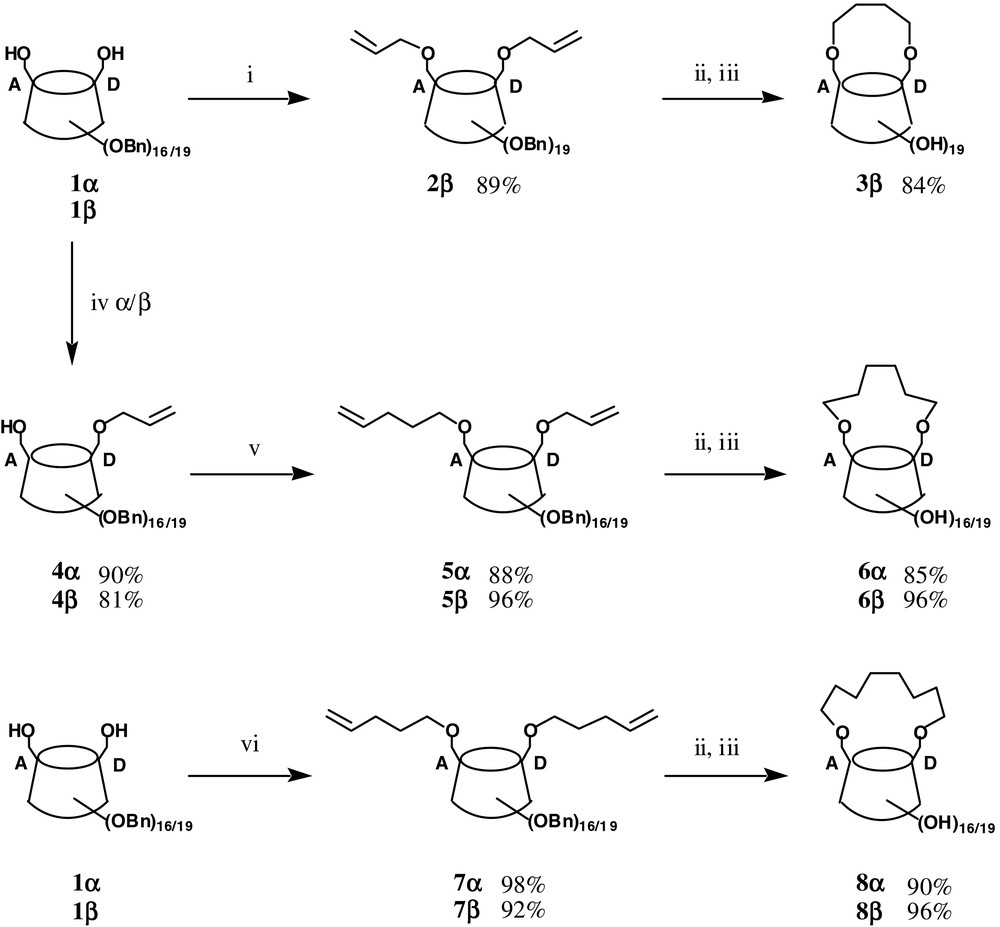
(i) AllylBr (4 equiv), NaH (4 equiv), THF, nBu4 NI (0.1 equiv), rt, 6 h; (ii) Cl2 (PCy3)2 Ru=CHPh (5 mol%), CH2 Cl2, reflux, 5 h then Pb(OAc)4 [4], rt overnight; (iii) H2, Pd/C 10%, Pd black, EtOAc/MeOH (1:1), 48 h, rt; (iv) for α-CD: AllylBr (1.1 equiv), NaH (2 equiv), THF, nBu4 NI (0.1 equiv), rt, 18 h; for β-CD: AllylBr (1.1 equiv), KH (1.1 equiv), THF, nBu4 NI (0.2 equiv), rt, 18 h (v) 5-bromo-pent-1-ene (4 equiv), tBuOK (4 equiv), nBu4 NI (0.1 equiv), THF, rt, 8 h; (vi) 5-bromo-pent-1-ene (8 equiv), tBuOK (8 equiv), nBu4 NI (0.1 equiv), THF, rt, 8 h.
The synthesis of the 6A,6D-hexamethylene-bridged CDs 6α and 6β was next achieved as shown in Fig. 2. A key feature is the possibility to perform a high-yielding mono-O-allylation of either 1α or 1β to provide 4α (90%) and 4β (81%), respectively. This opens the door to the preparation of various oligomethylenic capped CDs with odd or even carbon atom numbers. As an example, pentenylation of the alcohol 4α, followed by Ring Closing Metathesis (RCM), then hydrogenolysis, gave the capped CD 6α. Selected data for 6α: [α]D 20 = +121 (c = 0.2, MeOH); MS (MALDI-TOF): m/z (%): 1077.3 (100) [M + Na+]; 13 C NMR (100 MHz, D2O): 102.2, 101.8, 101.7 (3 × C1), 29.2, 25.8 . A similar sequence provided 6β. Selected data for 6β: [α]D20 = +123 (c = 0.2, MeOH); MS (MALDI–TOF): m/z (%): 1239.5 (100) [M + Na+]; 13C NMR (100 MHz, D2O): 102.6, 102.4, 102.3, 102.2, 102.1, 102.0, 101.8 (7 × C1), 29.5, 29.2, 25.7, 25.4 , . This product has also been recently prepared by another route [3], then converted to the per-O-methylated derivative, which was also able to separate enantiomers of particularly large molecules, including pharmaceuticals of different structural types.
Selected data for 4α: [α]D20 = +34 (c = 1, CHCl3); MS (FAB): m/z (%): 2477.0 (100) [M + Na+]; 13C NMR (100 MHz, CDCl3): 134.5 , 116.9 , 98.7, 98.4, 98.1, 98.0, 97.9, 97.8 (6 × C1), 61.2 .
Selected data for 4β (obtained as an inseparable mixture of isomers): MS (FAB): m/z (%): 2910.2 (100) [M + Na+]; 13C NMR (100 MHz, CDCl3): 134.64, 134.57 , 116.8 , 98.83 (1 × C1), 98.73 (2 × C1), 98.69 (2 × C1), 98.64, 98.57, 98.45, 98.37, 98.31, 98.16, 98.10, 97.89, 97.70 (9 × C1), 61.45, 61.38 .
Finally, the two 6A,6D-octamethylene bridged cyclodextrins 8α and 8β have been prepared as shown in Fig. 2. Selected data for 8α: [α]D20 = +120 (c = 0.15, MeOH); MS (MALDI–TOF): m/z (%): 1105.5 (100) [M + Na+]; 13C NMR (100 MHz, D2O): 101.9, 101.5, 101.4 (3 × C1), 29.2, 28.6, 25.4 . Selected data for 8β: [α]D20 = +125 (c = 0.2, MeOH); MS (MALDI–TOF): m/z (%): 1267.2 (100) [M + Na+]; 13C NMR (100 MHz, D2O): 102.7, 102.5, 102.4, 102.1, 102.0, 102.9, 101.7 (7 × C1), 29.6, 29.3, 28.7, 28.3, 25.8, 25.5 , , .
The capping of CDs had so far been achieved using tailor-made rigid aromatic disulfonylchlorides [4–16]. It is clear that the direct availability of diols 1α and 1β, combined with high regioselective mono-O-allylation and RCM methodology, provides a versatile entry to a family of oligomethylene-capped 6A,6D α- or β-cyclodextrins.
We have now studied the inclusion properties of p-nitro-phenolate (PNP) in 3, 6 and 8α/β. PNP derivatives are known to include with the nitro group close to the narrower rim [17], and to be very sensitive to the bulk of the guest [18]. The equilibrium constant is determined by UV–visible spectroscopy [19] in a phosphate buffer (pH = 11; I = 0.5). The concentration of PNP is 5 × 10–5 M and, even at the highest concentration of CD (5 × 10–3 M), all PNP is not bound. The values were therefore plotted according to the Hildebrand-Benesi [20] relation (equation (1)):
As reported in Table 1, the association constants for α- (log K = 3.24) and β-CD (log K = 2.80) are in agreement with the literature [21]. There is no inclusion of PNP with the shortly capped-CDs 3α/β. The 6A,6D-hexamethylene capped-CDs 6α/β show moderate association constants (log K = 2.83 and 2.66, respectively). Concerning the larger capped-CDs, 8α shows an enhancement of its association constant (log K = 3.95).
Association constants of PNP with AD-oligomethylene-capped-CDs 3,6 and 8α/β. NB = no binding
| log K | |
| α-CD | 3.24 |
| 3α | NB |
| 6α | 2.83 |
| 8α | 3.95 |
| log K | |
| β-CD | 2.80 |
| 3β | NB |
| 6β | 2.66 |
| 8β | 2.33 |
It thus appears that AD-oligomethylenic capping is able to modulate the complexing properties of cyclodextrins.
Acknowledgements
The authors would like to thank Dr J.-C. Blais (University Pierre et Marie Curie, Paris 6, France) for the MALDI–TOF mass spectra and Cyclolab (Hungary) for a generous supply of pure α and β-CD.



