1 Introduction
Since Vögtle and co-workers synthesized branched structures [1], dendrimers have generated enormous interest in diverse areas of science and technology owing to their precisely defined nanoscale, often globular, molecular structure [2]. Particularly, there has been an explosive growth in the interest in the chemistry of dendrimers incorporating transition metals into their structure, as part of the dendritic skeleton [3] or as peripheral functionalization [4]. Among the main potential applications of metalladendrimers, catalysis stands out as one of the most promising areas [5]. Indeed, these species offer a unique opportunity to combine the advantages of homogeneous and heterogeneous catalysis, given the possibility of recycling by means of nanofiltration [6]. Moreover, metalladendrimers may foster new approaches in catalysis as templates for the preparation and stabilization of monodisperse metal nanoparticles [7]. Although a number of dendritic backbones are known, carbosilane (CS) dendrimers are among the most widely used because of their thermal stability, catalytic inertness and accessibility. They are kinetically and thermodynamically highly stable molecules owing to the high Si–C bond dissociation energy (306 kJ mol–1), very similar to that of C–C bonds (345 kJ mol–1) and to the low polarity of the Si–C bond. In addition, most of the known CS dendrimers exhibit high flexibility, manifested by low glass transition temperatures. Therefore, it is not surprising that a good number of metallacarbosilane dendrimers have been explored to date [8–10]. This article focuses on the efforts made in this area during the last ten years.
Metallacarbosilane dendrimers are formed by grafting metal units on the core or the surface of CS dendrimers. In fact, only two papers concerning the first case have been published and they describe some palladium– [11] and rhodium-containing [12] dendrimers. Thus, the vast majority of metallacarbosilane dendrimers have been synthesized by anchoring metal units on the periphery of the dendrimer, which should exhibit an appropriate terminal group for the link. Although phosphine ligands have been the most used ones, other functions are also useful. Therefore, this paper is structured in two main sections: metalladendrimers from (a) CS with terminated groups other than phosphines and (b) CS-containing peripheral phosphine ligands. A brief survey of CS dendrimers decorated on the surface with transition metal cluster fragments is also provided.
2 CS dendrimers with terminated groups other than phosphines
The high reactivity of Si–Cl bonds towards ferrocenyl-lithium and β–aminoethylferrocene has facilitated the synthesis of a number of ferrocenyl-containing dendrimers [13–15]. Some of them [16] show electronic interactions between the two ferrocenyl units linked by the bridging silicon. In addition, some species have been used as mediators in amperometric biosensors [13] (Fig. 1).
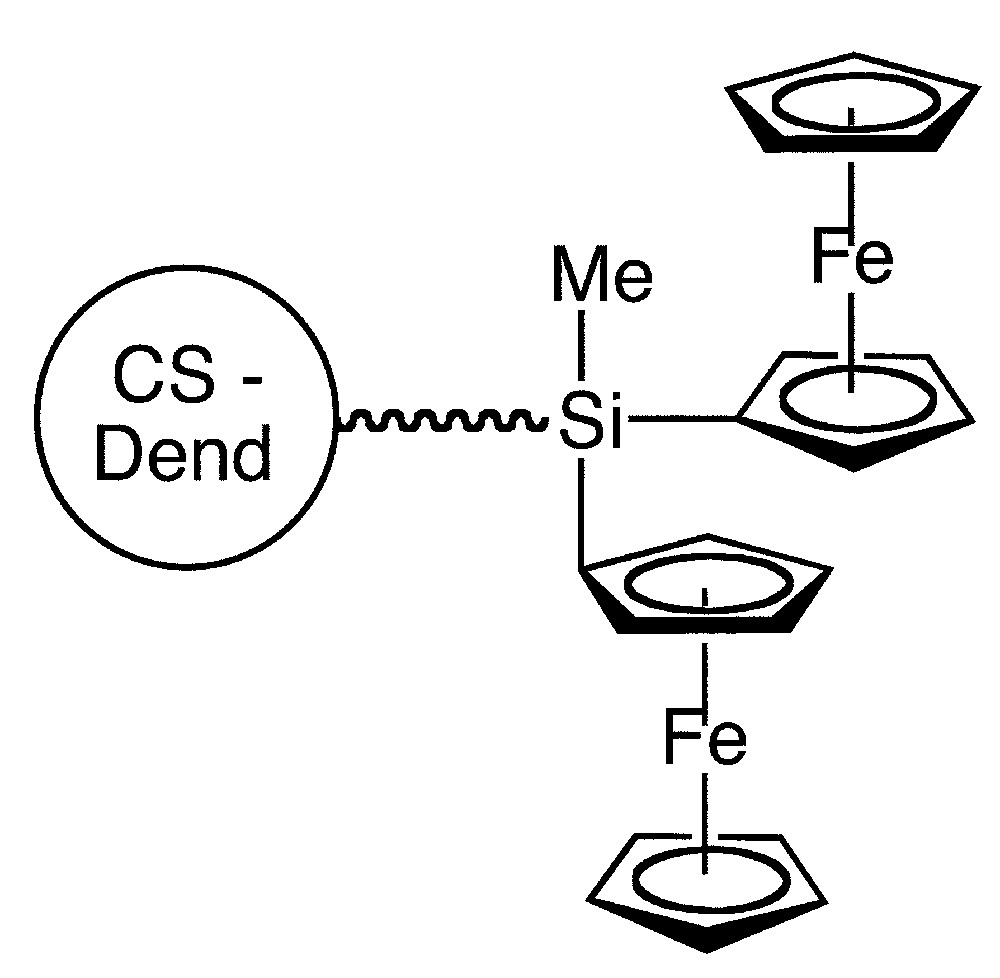
Remarkably, ferrocenyl functionalized dendrimers recognize and sense anionic guests, such as HSO4– and H2PO4–, via significant cathodic perturbations of the oxidation potential of the ferrocene couple [17].
A series of highly charged carbosilane dendrimers were obtained by reaction of [Cp*Ru(NCMe)3]+OTf– (Cp* = C5Me5, OTf = O3SCF3) with benzyl-terminated dendrimers. The polycations containing charges of 12+, 24+, 36+ and 72+ have been characterized by NMR spectroscopy and MALDI-TOF spectrometry [18] (Fig. 2).

Following a similar approach, a family of macromolecules containing η6-coordinated Cr(CO)3 moieties at the periphery of CS dendrimers was prepared by a process involving thermal replacement of CO from chromium hexacarbonyl by phenyl-terminated dendrimers [19, 20]. Cyclic voltammetric studies of the zeroth generation revealed that tricarbonylchromium moieties are non-interacting redox centres.
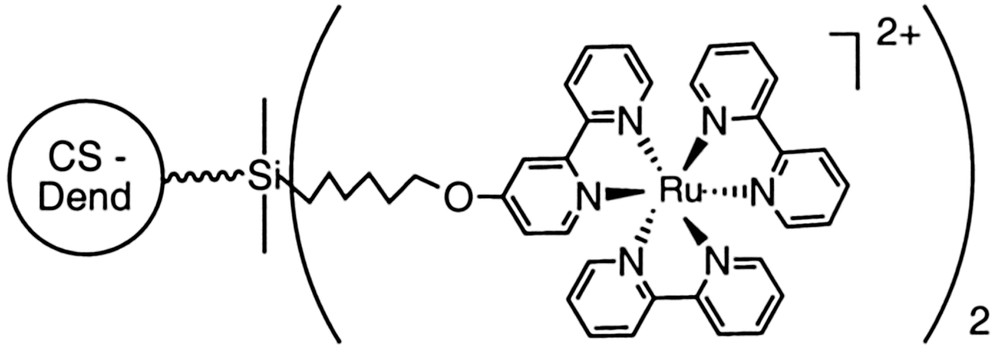
Eight peripheral ruthenium(II) tris(bipyridine) units covalently linked to the dendrimer were obtained by addition of 4-chloro-2,2′-bipyridine to a CS containing eight OH terminal groups, followed by the addition of Ru(bipy)2Cl2·2 H2O. Photophysical and electrochemical studies confirmed that the identical [Ru(bpy)3]2+ units are non-interacting [21] (Fig. 3).
Reaction of Co2(CO)8 with all the C≡CH groups in a series of CS dendrimers resulted in the synthesis of acetylenedicobalt hexacarbonyl-containing dendrimers. The structure of Si[CH2CH2SiMe2–C2HCo2(CO)6]4 was determined in an X-ray diffraction study [22].
We would like to underline that the reaction of dicobalt octacarbonyl with a dendrimer possessing two ethynyl substituents on the same arm of the dendrimer failed because of steric factors. Later, Kim et al. reported similar type of materials, but unlike Seyferth et al., they reacted dicobalt octacarbonyl with dendrimers possessing two phenylethynyl substituents on each peripheral silicon atom [23].
Amine functionalized carbosilane dendrimers were the starting material for the synthesis of the first metal-carbene-containing dendrimers. They were obtained in situ by the reaction of several generations of CS dendrimers with Ni(CN–tBu)4(ClO4)2 [24]. These species were used as initiators in the polymerization of isocyanides. W– and Ru-carbenes combined with low generation CS dendrimers have been tested as metathesis initiators [25] [26]. They show very high activity for the ring opening metathesis polymerization of norbornene.
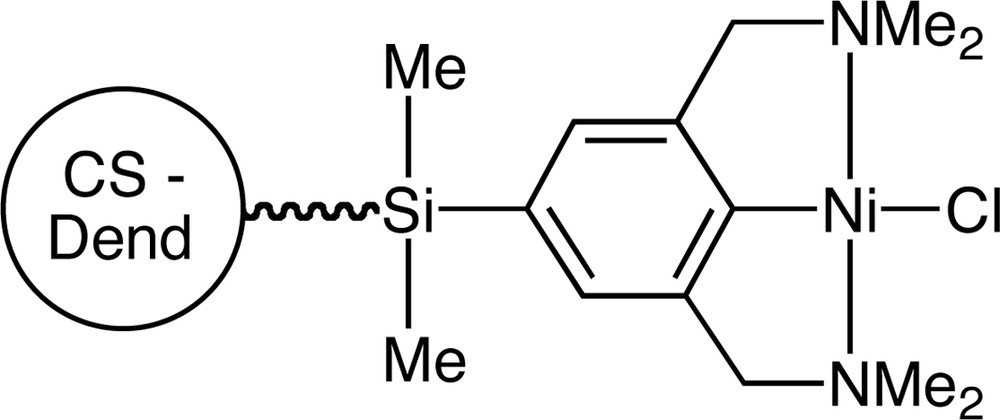
New families of species have been described by van Koten’s group by attaching [NCN]– chelating aryldiamine ligands, such as [2,6–CH2NMe2}2C6H3]–, and their phosphorous analogues, bisphosphinoaryl [PCP′]–, to the exterior of carbosilane dendrimers. These species have shown strong propensity to form stable transition metal complexes [27–31], which are effective catalysts for the synthesis of fine chemicals. For example, nickel-containing CS dendrimers have been used as catalysts in the Kharasch addition of polyhalogenoalkanes to carbon-carbon double bonds [32] (Fig. 4).
A series of cycloplatinated dendrimers appended with the monoanionic C,N–bidentate ligand [C6H4{CH2NMe2}2]– were also prepared via a multiple C–H activation reaction [33].
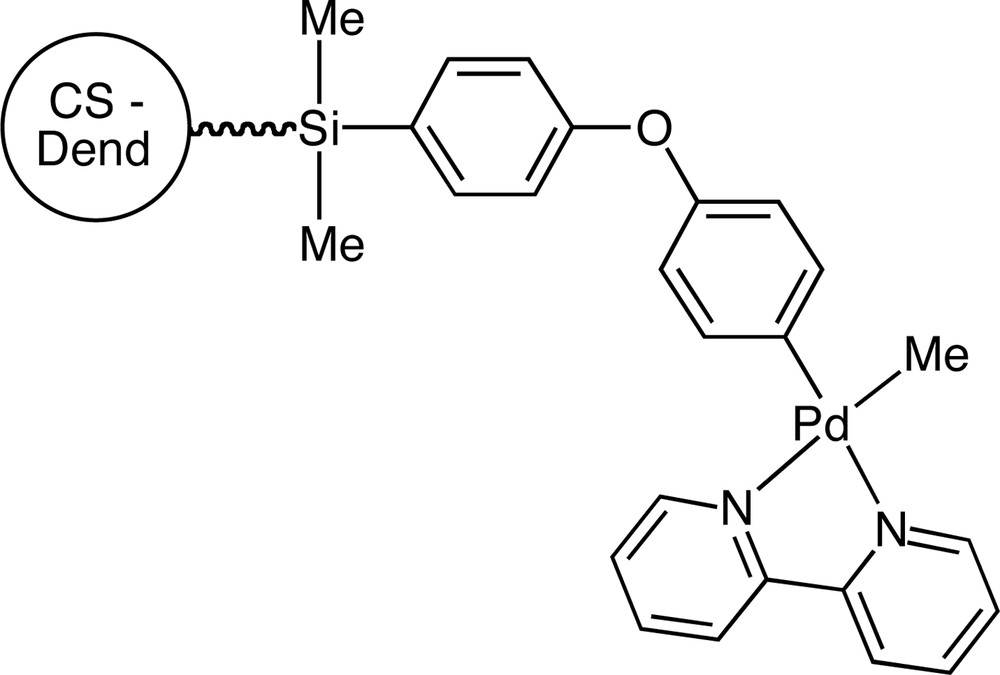
A carbosilane dendrimer containing 12 peripheral iodoarene groups, [Si{(CH2)3Si((CH2)3SiMe2(C6H4CH2OC6H4I–4)}] was treated with [Pd2(dba)3·dba/tmda] (dba = dibenzylideneacetone, tmda = N,N,N′,N′–tetramethylethylenediamine) to yield a periphery palladated complex which reacted with LiMe and 2,2′–bipyridine to form an organopalladium-containing dendrimer. This compound underwent oxidative addition with benzyl bromide to form species containing Pd(IV) centres [34] (Fig. 5).
The hydrovinylation of styrene in a membrane reactor was catalyzed by palladium complexes using dendrimers with hemilabile P–O ligands. The new dendritic Pd-catalysts are stable in the reaction conditions, whereas similar complexes of monodentate ligands are susceptible to decomposition, namely to the formation of palladium black [6, 35] (Fig. 6).

Palladium ion-containing CS dendrimers were prepared by the addition of palladium dichloride to double-layered dendritic carbosilanes, consisting of phenylethynyl groups on the outmost periphery and phenylethenyl and propyleneoxy groups in the inner shell, and their conducting behaviour was measured [36].
Some examples of early transition metal-containing CS dendrimers have also been described. For example, the treatment of dendrimers containing SiMe2(CH2)3{C6H3(OMe)(OH)} terminating groups with [CpTiCl3] provided an effective route for the attachment of cyclopentadienyl titanium complexes to the dendritic periphery [37, 38] (Fig. 7)

On the other hand, other titanium units have been grafted on the surface of a para-aminophenoxy terminated dendrimer [39].
A series of titanium and zirconium metallocenes bearing one or two first-generation silane dendritic wedges as bulky substituents at their cyclopentadienyl rings were synthesised by E. de Jesús et al. [40]
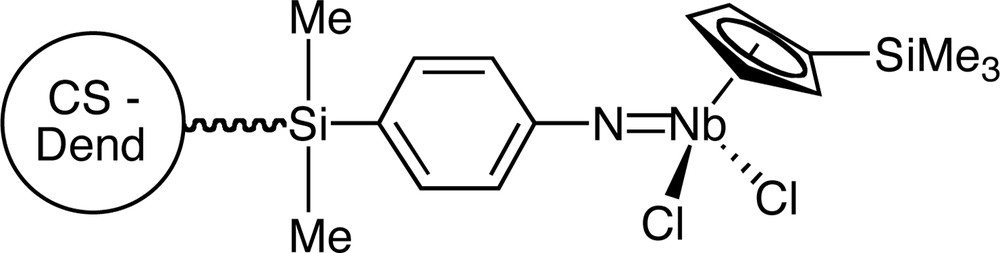
The N,N–bis(trimethylsilyl)aniline terminated groups of a series of carbosilane dendrimers reacted with niobium pentachloride or [NbCp′Cl4] to give species in which the metal moieties are linked to the framework through imido-metal bonds [41] (Fig. 8).
3 CS dendrimers with phosphine terminated groups
The general method to anchor alkyl- or arylphosphine groups on the surface of the CS dendrimer is the nucleophilic substitution of the chlorine atom of the Si–Cl functionalized dendrimer. Lithium methyldiphenylphosphine–TMEDA is the preferred nucleophile [42, 43], although LiCH2P(CH3)2 and LiCH2P(C6H13)2 [45], among others, have also been used. In general, they are obtained as wax-like solids. Characterization by 1H and 31P{1H} NMR spectroscopy reveals that the 31P-functionalised dendrimers obtained following this strategy show at least 95% purity. However, surface congestion precludes the synthesis of species containing a high number of phosphine groups [44, 45].
The presence of phosphine ligands on the surface of dendrimers has allowed the anchoring of a group of transition metal units by known strategies in coordination chemistry, e.g. by reaction with dinuclear halide bridged complexes or through ligand displacement reactions. The first strategy has been widely employed for palladium dendrimers and more recently for rhodium and iridium compounds, while the second has been used for platinum and gold derivatives.
Thus, a series of CS dendrimers containing monodentate or bidentate chelating phosphine ligands were reacted with [(η3-C3H7)PdCl2]2 to give allyl palladium complexes of these dendrimers, which were used as catalysts in the allylic alkylation of allyl trifluoroacetate and sodium diethyl methylmalonate to yield diethyl allylmethylmalonate [42]. All dendrimeric catalysts showed very high activity. Moreover, the higher generations are sufficiently large to be retained by a nanofilter, and they have been applied in a continuous-flow membrane reactor [46]. The stability of palladium complexes is crucial for application in such type of processes and appears to be very sensitive to small changes in the dendrimeric structure. A similar series of CS palladium dendrimers were tested as catalysts in the hydrovinylation of styrene, and their activity was compared with that of the model catalyst [PdCl(η3-2-MeC3H4)(PPh2CH2SiMe3)] [47].
Convenient synthetic routes to new functionalized diphosphines suitable for catalyst immobilization on carbosilane dendrimers have been reported by Gade et al. [48]. Fixation has been achieved for the simplest case of zeroth generation dendritic systems, which reacted with [(COD)PdCl2] and [(PhCN)2PtCl2] to afford the corresponding metallated systems.
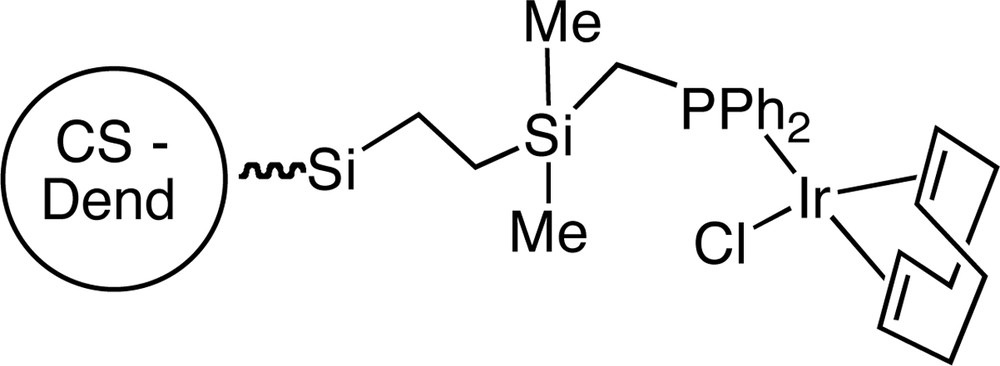
Following the same methodology, our group has recently described the synthesis of some rhodium- or iridium CS dendrimers containing MCl(cod) moieties on the surface by the reaction of phosphanyl-terminated dendrimers with [MCl(cod)]2 (M = Rh, Ir; cod = cyclooctadiene) [49] (Fig. 9).
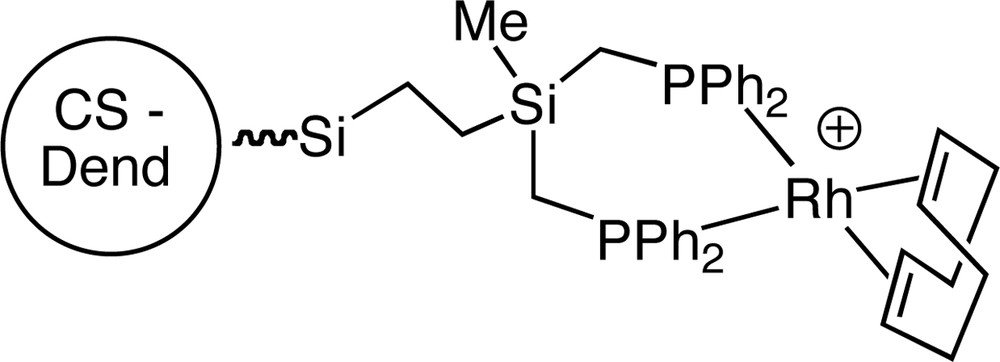
The same reaction with dendrimers displaying two phosphorous ligands per arm gave very unstable species that were not isolated. However, they reacted with [MCl(cod)]2 in the presence of silver salts to give the moderately stable cationic metalladendrimers shown in Fig. 10.
A bimetallic Rh/Fe layer-containing dendrimer was formed by substitution of cod by 4,4-bis(diphenylphosphino)ferrocene (dppf) ligand (Fig. 11).

The new metalladendrimers were tested as catalysts in the hydrogenation of 1-hexene. In general, cationic rhodium dendrimers are less active than the corresponding monomeric Rh(I) species [Rh(cod)(dppp)][CF3SO3], but show higher activity than the neutral dendritic species of the same generation.
An excellent route to prepare functionalized CS dendrimers involves the reaction of the CS dendrimer with the p–lithiated bromobenzene, followed by reaction with ClPR2 (R = Ph, Cy) [50]. Thereafter, such species have been used to anchor a Ru(II) complex upon reaction with an N2–bridged binuclear complex, [{RuCl2(η3–N,N′N)}2(μ-N2)] (NN′N = 2,6–bis[(dimethylamino)methyl]pyridine).
Rhodium complexes of alkylphosphine-containing dendrimers based on polyhedral oligosilsesquioxane core were prepared in situ using [Rh(acac)(CO)2] or [Rh2(O2CMe)4] as metal source [44, 51]. The 31P NMR study of their solution revealed a complicated mixture of species in solution. They were used as catalysts for the hydrocarbonylation of hex-1-ene, oct-1-ene, non-1-ene and prop-1-en-2-ol [52, 53]. Dendrimer generation did not modify the selectivity of hydroformilation, but the number of functional groups and the length of the bridge between phosphines conditioned the reactivity of rhodium complexes [44].
An efficient strategy for the backbone functionalization of a tripodal phosphine ligand that allows its attachment to carbosilane dendrimers has recently been developed [54]. Thus, CS dendrimers containing OCH2CH2Otriphos functions were prepared and metallated with [Rh(COD)2][BF4] or [Mo(CO)3(MeCN)3], selectively yielding the metallated dendrimers shown in Fig. 12.

The catalytic properties of the rhododendrimers in the hydrogenation of styrene and 1–hexene were examined and compared with those of the mononuclear model compound [Rh(triphos)(COD)][BF4].
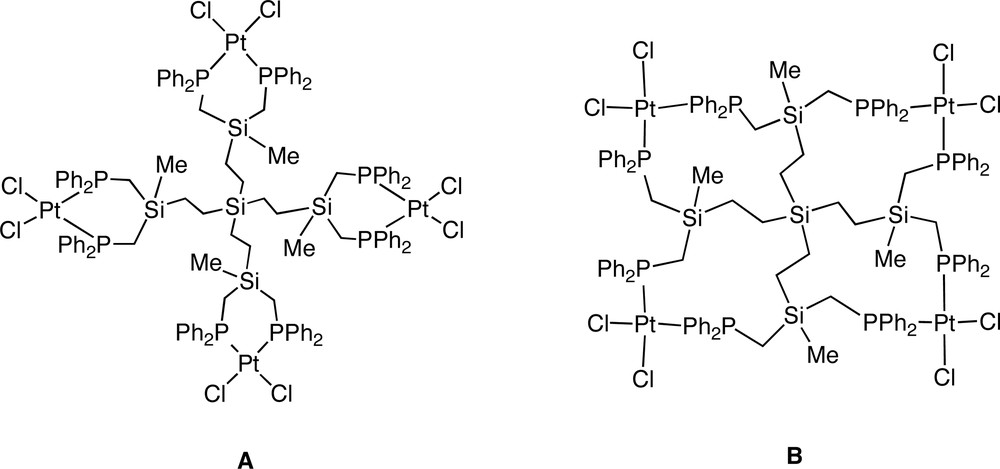
Platinum CS dendrimers containing PtCl2 as peripheral groups were synthesised by the reaction of peripheral phosphine dendrimers with [PtCl2(cod)] [47]. Interestingly, the PtCl2 units can be bound on the surface of the dendrimer through two P atoms belonging to the same branch, or to distinct branches. On the other hand, the unique signal in the 31P NMR spectrum revealed that one only isomer was formed. By comparison with the NMR data of two model compounds, the isomer formed was assigned to the stereoisomer A (Fig. 13).
Dendrimers containing platinum acetylide units were prepared similarly by reaction of [PtCl2(C≡CPh)2] with the phosphine functionalized CS dendrimer. The new radially metal-layered dendrimers are relevant because of their potential use in catalytic processes and as precursors of cluster-containing dendrimers. Indeed, they were used to form multiple {C2Co2(CO)6} cluster sites [47] (Fig. 14).

Gold-containing CS dendrimers [43, 55] were prepared following the synthetic approach described by Majoral and co-workers [4], consisting of the reaction of AuCl(tht) (tht = tetrahydrothiophene) with phosphine-ended CS dendrimers. This process takes advantage of the lability of the tht ligand. The reaction is clean and yields about 90%. It is easily monitored by 31P NMR spectroscopy, which shows total disappearance of the signal of the starting CS dendrimer while new signals emerge owing to the gold dendrimers.
4 Transition metal clusters-containing CS dendrimers
The lability of the chloride ligand attached to the gold atom has allowed the formation of mixed transition metal clusters by reaction of ClAu(phosphine) with a number of anionic polymetallic complexes. For example, metal clusters exhibiting FenAu (n = 2–6) cores have recently been synthesized [56]. Following this approach, we reacted [Si(CH2CH2SiMe(CH2PPh2AuCl)2)4] with [Fe3(CO)11]2– and [PPh4]8[Si(CH2CH2SiMe(CH2PPh2{AuFe3(CO)11})2)4] was obtained as a very insoluble material. To increase the solubility of the resulting species, we modified our initial approach in the following terms: (i) by the use of mononegative iron anions, which can afford neutral compounds, generally more soluble and (ii) by enlarging the dendritic structure with the incorporation of CH2CH2SiMe2 units as spacers but keeping the same number of phosphine functions to decrease surface congestion and charge density [57]. Our expectations were confirmed with the formation of soluble clusters that were spectroscopically characterized (Fig. 15).

Given the successful use of the Au–Cl in the formation of iron/gold clusters-containing CS dendrimers, we extended this reaction to other metal systems to corroborate the applicability of the method.
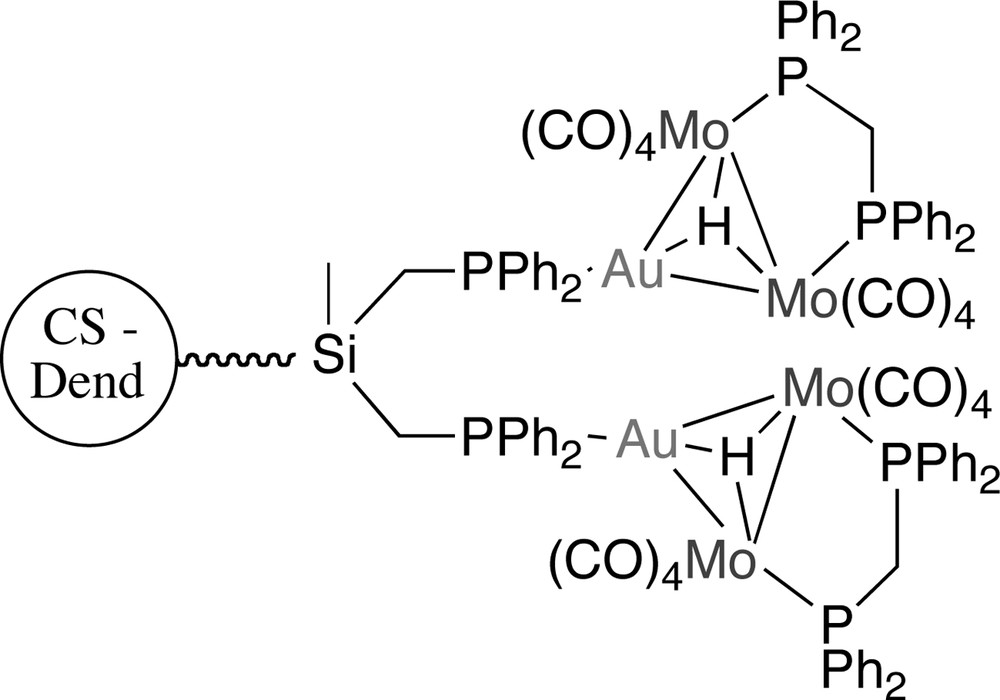
Thus, two carbonylmetallate anions of molybdenum were employed: the sodium salt of [MoCp(CO)3]– and [NH4][Mo2(μ-H)(μ-dppm)(CO)8] (dppm = diphenylphosphinomethane) [45]. In the first case, the reaction proceeded rapidly and a family of new dinuclear Au/Mo units grafted on the surface of the CS dendrimers was cleanly obtained. The displacement of the chlorine atom by the dinuclear hydrido Mo2 fragment required the presence of thallium salt as a halide abstractor. The reaction was instantaneous and the soluble products were obtained as air-stable yellow solids and characterized by multinuclear NMR spectroscopy (Fig. 16).
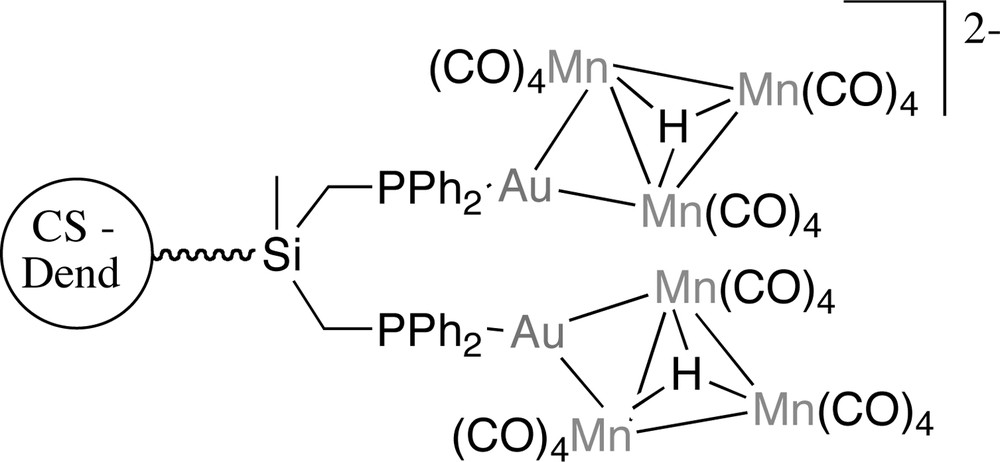
The manganese carbonyl anion used for obtaining Au/Mn cluster units attached to the CS dendrimers was [Mn3(μ-H)(CO)12]2–. Its tetraphenylphosphonium salt reacted with the gold dendrimer in an hour and gave good yields of soluble dark-green compounds that were air-stable solids. Although no parent ion was observed in MALDI–TOF–MS, the cluster/dendrimers were unambiguously characterized by NMR spectroscopy [45] (Fig. 17).
5 Conclusions
CS dendritic macromolecules containing metal fragments on their surface offer attractive advantages since, besides their thermodynamic stability, they possess a precisely defined molecular architecture that can be achieved by rational synthetic procedures (Tables 1–7). This opens the possibility of modulating the nuclearity of the resulting macromolecules to obtain desirable properties, not only in the field of homo– and heterogeneous catalysis, but also in medicine, pharmaceutical applications and the preparation of novel materials.
Acknowledgements
We thank DGCYT and CIRIT for continued financial support.


