1 Introduction
Metallodendrimers [1–12] are now a well-established field of research due to the efforts of both communities of dendrimer and inorganic chemists. They potentially provide ecologically viable and regenerable catalysts whose efficiency can be equal to those of mononuclear homogeneous catalysts and which can be easily recovered and re-used. They are also ideal hosts for supramolecular chemistry [13] and, as such, have been designed as exo-receptors for sensing and titration of biologically relevant anions [14]. Finally, they can be decorated with redox-active transition-metal groups at the periphery, which provides a mean to exchange a large number of electrons at virtually the same potential [1] for purposes involving electronic, photonic, magnetic or multifunctional devices. In the present article, we will give examples of our efforts towards the achievement of these goals and introduce them in the literature context. We will also compare dendrimers with nanoparticles [15–20] and especially to a new family, the dendronized thiolate-gold nanoparticles.
2 Construction of giant dendrimers
The organo-iron activation of simple or functional arenes such as mesitylene and p–ethoxytoluene by the 12–electron fragment CpFe+ [21] has provided dendritic cores and building block (dendrons) for the construction of giant dendrimers far beyond the De Gennes dense-packing limit [22] with numbers of branches up to more than 105. The construction consisted in iterating sequences of two reactions: (i) regioselective hydrosilylation of the terminal double bonds with chloromethyl dimethyl silane catalyzed by the Karsted catalyst and (ii) NaI-catalyzed nucleophilic substitution of the chloride by the phenoltriallyl building block [23] (Fig. 1). This strategy represents a variation of a previously reported one involving hydroboration using the bulky disiamylborane [24] instead of hydrosilylation. The use of these bulky boranes had led to a steric limitation at the third generation that is no longer observed in the present scheme. Each single reaction of this divergent dendrimer synthesis could be followed by 1H, 13C and 29Si NMR to insure completion until the ninth generation without visible defects on the NMR accuracy. The dendrimer structures were checked by MALDI–TOF mass spectrometry for the first generations, then by TEM (subsequent to vaporization of osmium oxide under a well-ventilated hood onto the poly-olefin dendrimers) and size exclusion chromatography (showing a polydispersity below 1.02 up to the fifth generation).
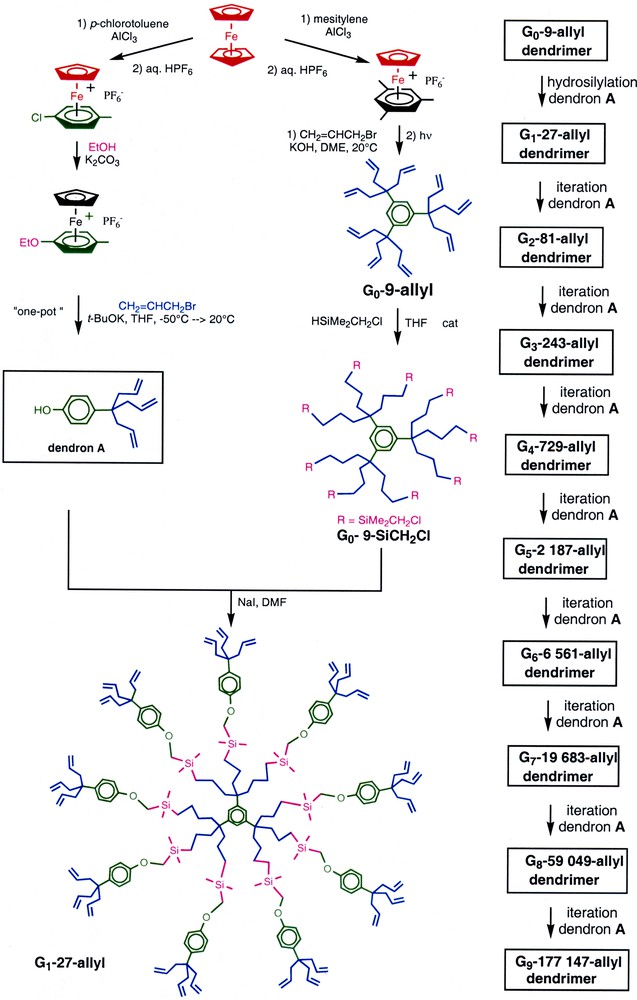
Strategy for the construction of large dendrimers starting from ferrocene.
The high-generation giant dendrimers were characterized by NMR (see above, solubility was without problem for instance in CDCl3), elemental analysis, TEM and by AFM showing the steady size progression up to a diameter of 13 nm for G9. The construction proceeded as usual with the appearance of defects, consisting, in the present case, in the lack of the phenoltriallyl building block (see the small side peak in the MALDI–TOF mass spectrum of G2–81–allyl in Fig. 2). In such high-generation dendrimers, it is probable that the branch termini turn inside the dendrimers towards the core in order to avoid steric congestion at the periphery, since the dense-packing limit (reached here around 3000 branches) is overtaken by far (this phenomenon may also intervene even below the generation corresponding to the dense-packing limit [24, 25]). This construction now serves as a basis for the various uses of dendrons, dendrimers and metallodendrimers in our laboratory.

MALDI–TOF mass spectrum of the G2-81-allyl dendrimer showing the dominant molecular peak and the small side peak corresponding to the lack of one substituted p–phenoxy group.
3 Metallodendritic redox and ROMP catalysis: stars vs. dendrimers in catalysis
The first dendritic catalysts have been designed by the groups of van Leeuwen [7, 8], Van Koten [9], Ford [26], Du Bois [27] and Brunner (dendrizymes [28]) in the early 1990s, and many reactions have been examined with dendritic catalysts recently [12]. We have engaged a research program with the goal of disclosing and studying various type of catalysis with metallodendrimers containing transition-metal fragments. Hexanuclear star-shaped, water soluble redox catalysts (see Fig. 3) for the cathodic reduction of nitrate to ammonia were found to show a kinetics of homogeneous electron transfer of their 19-electron FeI state to nitrate analogous to those of mononuclear catalysts with the same driving force [29] (this electron transfer follows a Marcus-type linear relationship indicating that it is the rate limiting step of the overall cathodic nitrate reduction).

Hexafunctionalization of a star compound with a heterodifunctional, water-soluble organometallic redox catalyst for the cathodic reduction of nitrates and nitrites to ammonia in water.
In contrast, dendritic and star-shaped cores containing the catalyst located at the center of the core react with nitrate at rates that are an order of magnitude slower, showing the dramatic effect of the strike bulk and thus, to a certain extent, some limit to the concept of efficient catalysis inside the dendrimer [30].
We subsequently designed metallodendrimers containing ruthenium–carbene fragments located at the dendrimer periphery on the branch termini [31–33] in order to perform Grubbs-type ROMP catalysis [34]. We therefore decorated dendrimers of generations 1 to 4 terminated with diphosphines [35, 36] using Hoveyda’s metathesis catalyst [32] as a starting point, which provided the four generations of new, stable metallodendrimers containing ruthenium-carbene fragments at the periphery (Fig. 4).

Strategy for the synthesis of organometallic dendritic stars starting from DSM’s dendritic amines and involving ROMP of norbornene.
The fourth-generation metallodendrimer containing 32 ruthenium-carbene fragments, however, was found to have a rather low solubility in common organic solvents, unlike the three first-generation complexes that respectively contained 4, 8 and 16 ruthenium-carbene fragments. The X-ray crystal structure of the model mononuclear complex in which the dendritic branch was replaced by a benzyl group showed the distorted square pyramidal geometry and the Ru=C double bond (Figs. 5 and 6). The three first generations of metallodendrimers and the model complex were efficient catalysts for the ROMP of norbornene. Analysis of the molecular weights by SEC gave data close to the theoretical ones, which indicated that all the branches were efficiently polymerized. Dendristars with an average of about 100 norbornene units on each dendritic branch were synthesized with the three first generations of ruthenium-carbene dendrimers containing respectively 4, 8 and 16 Ru=C bonds [37]. Two kinds of dendritic effect were found upon analysis of the kinetic data: (i) the dendrimers were more efficient catalysts than the model complex; (ii) the efficiency of catalysis decreased upon increasing the dendrimer generation. The first one could possibly be due to labilization of key metal-ligand bonds that is facilitated in dendrimers as compared to the monomer. The second one is probably related to the more difficult access to the metal center because of increasing steric effect at the dendrimer periphery when the generation increases.

ROMP of norbornene with the G3 dendrimer containing 16 ruthenium-carbene groups.
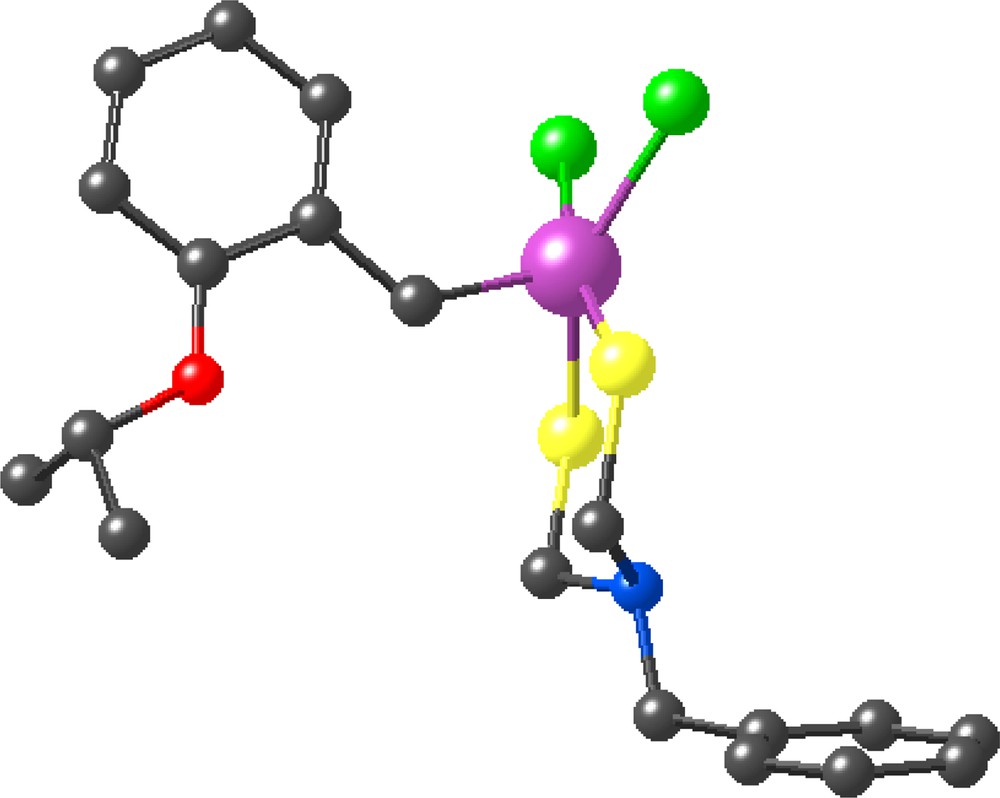
Simplified representation (without the two phenyl groups on each phosphorus atom) of the X-ray crystal structure of the model ruthenium-carbene complex in which the dendritic branch has been replaced by a benzyl group. Ru: purple; P: yellow; Cl: green; O: red; C: black.
4 A new kind of dendrimers: dendronized gold nanoparticles and their use as sensors for dihydrogenophosphate anion and ATP2–
Metallodendrimers can be assembled using classic covalent bond formations in a divergent or convergent way [1–10], but they can also be so using supramolecular chemistry involving hydrogen bonds [38, 39]. A new, original construction involves the assembly of dendrons onto gold nanoparticles giving stable nanoparticle-cored dendrimers [40–43]. The application of this strategy to tris-amidoferrocenylalkylthiol dendrons and to tris– and nonasilylferrocenylalkylthiol dendrons yielded gold nanoparticle-cored dendrimers containing up to about 200 ferrocenyl groups at the dendrimer periphery (Fig. 7).
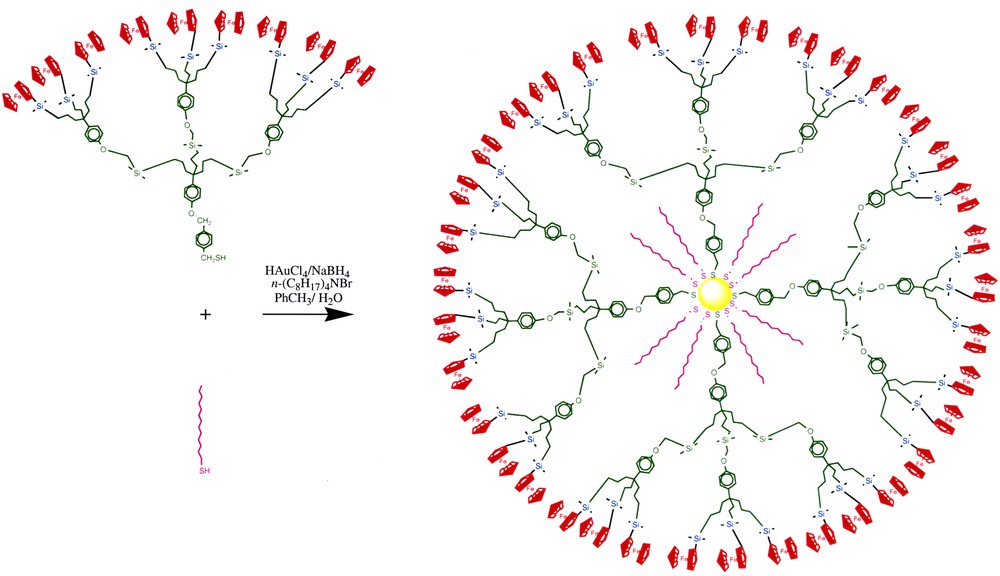
Assembly of stable dendronized gold nanoparticles.
These new dendrimers can selectively recognize oxo-anions and ATP2– using cyclic voltammetry (redox recognition by variation of the redox potential of the ferrocenyl group [44–47]) by synergy of:
- • (i) supramolecular interaction between the oxygen atom of the anion and the amido or silyl group conjugated with the ferrocenyl group;
- • (ii) electrostatic interaction between the oxo-anion and the cationic ferrocenium generated upon anodic oxidation;
- • (iii) topological effect at the dendrimer periphery whereby the small microcavities offer a narrow, selective channel for the supramolecular interaction. In the absence of this topology effect, the change of potential is very weak [48] (Fig. 8).
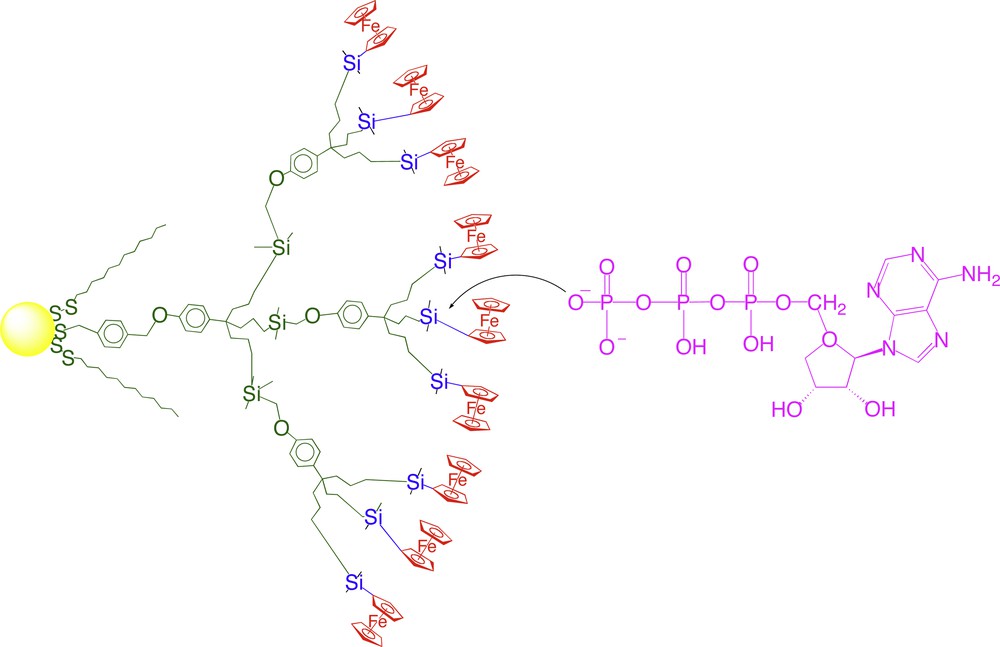
Interaction between the ATP dianion and the Lewis-acidic silicon atom of a ferrocenylsilyl branch of a part of a dendronized gold nanoparticle (see another representation of the dendronized nanoparticle in Fig. 7).
The selective recognition and titration of these oxo-anions can be effected in the presence of other less interactive anions such as hydrogenosulfate, chloride and the anion of the supporting electrolyte (BF4– or PF6– in large quantity). Gratifyingly, a platinum electrode can be modified with the dendrimer by scanning the ferrocenyl region to give very stable modified electrode that can be efficiently used as sensor. Upon washing this electrode with CH2Cl2, the ATP2– is removed and the original cyclic voltammetry wave of the dendrimer is recovered for repeated use. This operation can be carried out many times, because the supramolecular forces involved in the recognition are weak.
5 Metallodendrimers as molecular batteries in molecular electronics: towards nanotechnology
Metallodendrimers containing a large number of metal fragments that can provide robust redox systems at virtually the same redox potential are electron-reservoir systems that can be used as molecular batteries or in nano-devices for molecular electronics. For instance, it should be possible to connect two metal wires or two electrodes in a circuit involving such a large metallodendrimer as the molecular component.
We have synthesized dendrimers containing 54 ferrocenyl units using a precise convergent strategy [49] (Fig. 10) as well as other dendrimers resulting from divergent construction and containing theoretical numbers of 81 (Fig. 11) and 243 ferrocenyl groups [50] at the dendrimer periphery (Fig. 9).
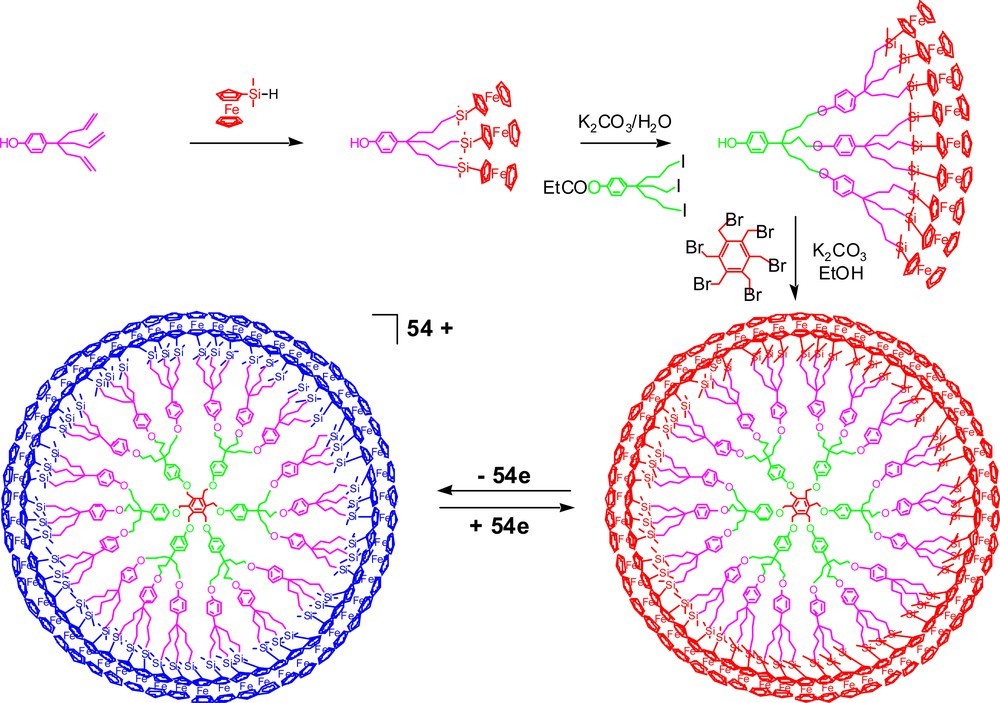
Convergent synthesis of a dendrimer containing 54 ferrocenyl groups and its reversible conversion to the blue ferrocenium analogue.

Divergent synthesis of a second-generation dendrimer containing a theoretical number of 81 ferrocenyl groups and its reversible oxidation to the stable ferrocenium dendrimer.
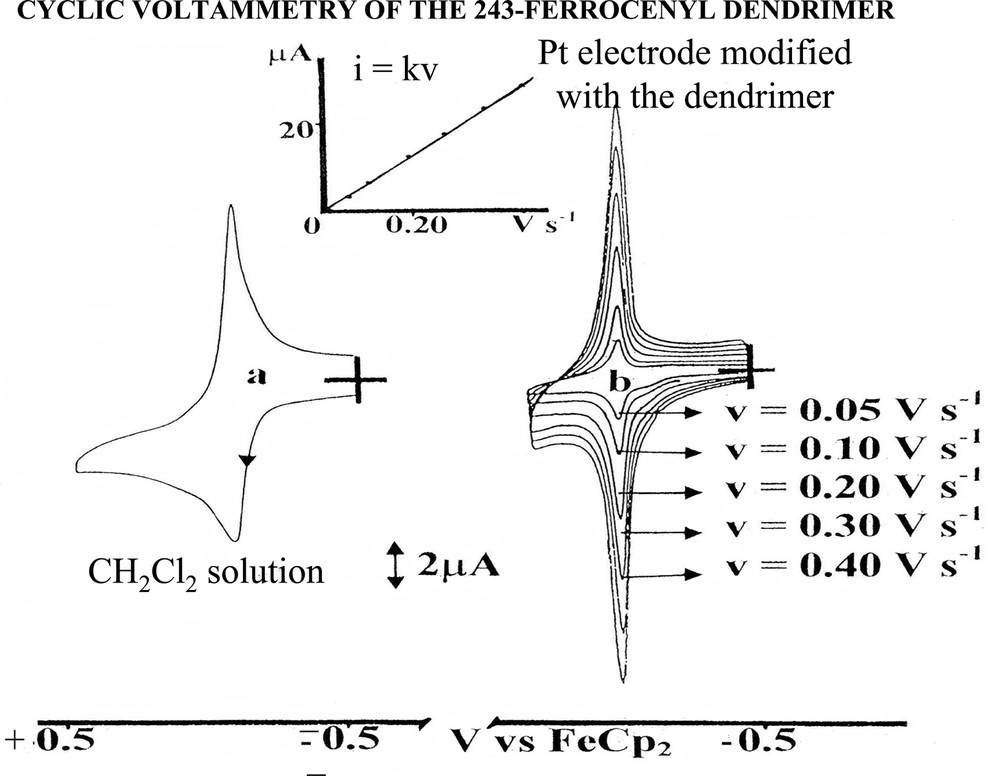
Cyclic voltammogram of the 243–ferrocenyl dendrimer (‘243–Fc’) in CH2Cl2 solution containing 0.1 M [n-Bu4N][PF6]: (a) in solution (10–4 M) at 100 mV s–1 on Pt anode; (b) Pt anode modified with ‘243–Fc’ at various scan rates, dendrimer-free clear CH2Cl2 solution (inset: intensity as a function of scan rate: the linearity shows the expected behavior of a modified electrode with a fully adsorbed dendrimer).
Using the giant dendrimers presented at the beginning of this article, it is now possible to construct dendrimers with considerably larger numbers of ferrocenyl groups. The ferrocenyl dendrimers that are already available can be oxidized anodically or using NOPF6 to blue ferrocenium dendrimers that are stable and have been characterized by Mössbauer spectroscopy as classic ferrocenium derivatives. These blue ferrocenium compounds can be reduced back to orange ferrocenyl dendrimers using decamethylferrocene. This cycle is effected quantitatively, i.e. this well-known ferrocene–ferrocenium redox couple is robust even in the dendrimers [50]. It is not an excellent electron-reservoir system, however, because the redox potential of the silylferrocenyl group is rather low. On the other hand, we have attached the excellent electron-reservoir system [FeCp(η6-C6Me6]+/0 (Cp = η5-C5H5) onto the commercial DSM polyamine DAB dendrimer G5-64NH2 (5th generation, theoretically 64 amine branches) using the chlorocarbonyl complex [FeII(η5-C5H4COCl)(η6-C6Me6)][PF6] to give the corresponding amido dendrimer dendr–{[(NHCOC5H4)FeII(η6-C6Me6)][PF6]}64. This dendrimer has been exergonically reduced to its deep-purple 19-electron FeI form using the prototype 19-electron, electron-reservoir complex [FeICp(η6-C6Me6]. The latter reacts with 64 equivalents of C60 by single exergonic electron transfer from each FeI site to C60 to give dendr–{[(NHCOC5H4)FeII(η6-C6Me6)][C60–]}64 that was characterized inter alia by its Mössbauer and EPR spectra [51] (Fig. 12).

Reduction of C60 to its mono-anion by a deep-blue 19-electron metallodendrimer containing a theoretical number of 64 FeI units (see a representation of the product in Fig. 13).
Note that all the ferrocenyl or metallocenyl dendrimers discussed in the present article present cyclic voltammograms in solution as well as on derivatized electrodes in which all the ferrocenyl groups appear equivalent in a single cyclic voltammetry wave. From these waves, it can also be concluded that the heterogeneous electron transfer is fast within the electrochemical time scale of this technique despite the large size of the ferrocenyl dendrimers [52, 53]. This means that the electrostatic factor [54] is very weak, presumably a fraction of a millivolt, because the ferrocenyl centers are sufficiently far from one another in all the dendrimers. Heterogeneous electron transfer is fast because the dendrimers rotate more rapidly than the electrochemical time scale in solution, which brings in turn all the ferrocenyl centers near the electrode [55, 56]. In the solid state, however, this reason cannot be invoked, because the dendrimers cannot rotate any more, their movements being considerably reduced. Thus, one must consider that fast electron hoping is occurring among the ferrocenyl groups within each dendrimer as well as among the ferrocenyl groups of different dendrimers. Indeed, multi-layers of ferrocenyl dendrimers are adsorbed onto the electrode surface by multiple scanning around the region of the potential of the ferrocene redox system, so that ferrocenyl dendrimer layers stack above one another as observed experimentally using the quartz micro-balance [18].
Comparison between multi-layers of ferrocenyl dendrimers of moderate size and adsorbed giant ferrocenyl dendrimers should soon allow to establish a comparison of the rate of electron hoping within a dendrimer and among dendrimers. Ultra-fast cyclic voltammetry might also help to provide an insight onto this kind of question by considerably reducing the electrochemical time scale and meanwhile the diffusion layer.
6 Conclusion and prospects
Access to new nano-sized dendrons, dendrimers, stars, dendristars, supramolecular dendritic assemblies and dendronized nanoparticles has provided us a variety of molecular nano-materials with properties as catalysts, sensors, molecular batteries and components of nano-devices for molecular electronics. A very large field of applications is open from molecular biology [57, 58] (gene therapy) to recoverable catalysts for green chemistry [7–9] and forthcoming nano-technology [59]. We are now embarked in research programs towards such applications involving various collaborations.


