1 Introduction
The valorisation of vegetable product represents a good alternative as opposed to the ineluctable exhaustion of the petroleum reserves. Principal qualities of the natural substances are their low ecotoxicity, their great biodegradability, and moreover these resources are renewable and very diverse. In this field, vegetable oils are largely used into agro-alimentary industry, but still little at the industrial level. Indeed, in spite of efforts of research, these oils still contain too many unsaturated fatty acids (C18:2 and C18:3), very sensitive to oxidation, which limits their uses in the non-food sector. The oxygen absorption rate of the linolenic acid (C18:3), the linoleic acid (C18:2) and the oleic acid (C18:1) is 800:100:1, respectively. The oleic acid C18:1 in the ‘cis’ isomer form has not only the advantage of being stable under oxygen atmosphere to avoid polymerisation, but moreover it remains liquid at low temperatures. So, the applications are as varied as pharmacy, cosmetics, plastics, detergents, and lubricants... But these industrial needs require a minimum of 80% in oleic acid, while traditional sunflower oil contains only 25 to 30%. Currently, the catalysts used at the industrial level for the hydrogenation of the fatty acids mainly contain nickel. Studies on the hydrogenation of different vegetable oils have been also undertaken on catalysts containing copper [1] or copper chromites [2]. These various systems have good selectivity, but remain however not very active even at the high reaction temperatures used. The aim of the present work is to develop catalysts of hydrogenation containing low loadings of noble metals.
We studied the selective hydrogenation of a C=C double bond of the C18:2 molecules of sunflower oil ethyl esters, while trying to avoid formation of C18:1 trans isomer and total hydrogenation towards stearic C18:0. Selective hydrogenation was carried out at low temperature (40 °C) on ethyl esters of traditional sunflower oil in the presence of supported catalysts containing noble metals Pd, Pt or Ru. In order to improve the selectivity in cis oleic derivatives C18:1, these catalysts were modified by inactive metals or by addition of amines into the reaction medium.
2 Experimental
2.1 Catalyst preparation
The support (Degussa Aerosil silica, surface area 200 m2 g–1) was ground and then sieved to retain particles with sizes between 0.10–0.04 mm. The preparation of the monometallic Pd/SiO2, Pt/SiO2 and Ru/SiO2 catalysts was carried out by impregnation of the support by the precursor salts (PdCl2, H2PtCl6 and RuCl3, respectively) in basic medium (pH = 11, cationic exchange). Monometallic catalysts were calcined in flowing air for 4 h at 300 °C, then reduced in flowing pure hydrogen for 4 h at 500 °C (Pd and Pt catalysts) or 400 °C (Ru catalyst). For the three monometallic catalysts, the size of most metal particles is around 2.0 nm according transmission electron microscopy (TEM) studies.
Bimetallic Pd–Cu catalysts were prepared by surface redox reaction between hydrogen activated on palladium particles and the copper salt (Cu(CH3COO)2) dissolved in water (‘catalytic reduction’ method [3]). This preparation method of bimetallic catalysts is well known to induce a strong interaction between the two metals whatever the prepared bimetallic system [4–6]. A known amount of the pre-reduced palladium catalyst was introduced into a reactor under nitrogen and was activated at 300 °C for 1 h under hydrogen. Then the degassed solution of the modifier precursor in water was introduced onto the catalyst at room temperature. After 90-min reaction time under hydrogen bubbling, the solution was filtered out and the catalyst was dried overnight at 100 °C. Bimetallic Pd–Pb catalysts were prepared also by catalytic reduction with Pb(CH3COO)2 as precursor salt, in methanol as solvent and under hydrogen bubbling during 1 h. Finally, all the bimetallic catalysts were reduced under hydrogen flow at 300 °C or 500 °C for 2 h (2 °C min–1 heating rate). Blank palladium catalysts were prepared following the same procedure, but the solution containing the modifier salt was replaced by a methanol or an aqueous solution.
The elemental analysis of metal contents in the catalysts was determined by the atomic absorption method.
2.2 Hydrogenation of sunflower oil
Classical refined sunflower oil (from Valagro) was transformed in presence of ethanol and sodium ethanolate, under 60 °C, into glycerol and ethyl esters. After the separation of the reaction products by decantation, the sunflower oil ethyl esters (SOEE) were washed and dried at 110 °C for 3 h under vacuum. They were then purified on a molecular distillation apparatus at 120 °C (P < 10–3 mbar) until a high purity near 98%. The hydrogenation of SOEE was performed in liquid phase in a stirred autoclave, at constant pressure of 10 bar and at low temperature (40 °C) according to an experimental procedure previously implemented in the laboratory. A known amount of pre-reduced catalyst was immersed into 50 ml of solvent (ethanol) without exposure to air and introduced into the autoclave. Then a mixture of 75 ml of SOEE in 25 ml of solvent was poured into the autoclave and flushed with nitrogen. When the desired temperature was raised, a 10-bar hydrogen pressure was introduced and the stirring was switched on (zero time for the reaction). Some experiments were also carried out with introduction of a known amount of amine in the reaction medium. Liquid samples were analysed by VPC on a Varian 3400CX chromatograph equipped with a flame ionisation detector and a capillary column DB-23 (J&W, 30 m, 0.25 mm i.d.), using nitrogen as carrier gas.
3 Results and discussion
All the prepared catalysts and the results obtained in SOEE hydrogenation are reported in Table 1.
Hydrogenation of SOEE in the presence of different catalysts supported on silica (T = 40 °C, PH2 = 10 bar, metallic concentration in the reaction medium = 0.077 × 10–3 mol l–1 [* 0.230 × 10–3 mol l–1])
3.1 Monometallic catalysts
First, the three monometallic catalysts Pd, Pt and Ru were compared (entries 1–3). For similar particle size (around 2.0 nm), the Pd catalyst shows the best activity, since a 95% conversion is reached after a 13-min reaction time, while Pt and Ru need longer durations and higher catalyst weights for the same conversion to be obtained. The order of the activities Pd > Pt > Ru observed here is the same one as that described elsewhere [7,8]. On the other hand, the three metals lead to the formation of significant amounts of trans oleic C18:1 and stearic C18:0 derivatives. However the Pd catalyst is the most selective to oleic molecules.
3.2 Bimetallic catalysts
In order to inhibit the formation of stearic derivatives and to improve the selectivity to cis oleic C18:1, the 1-wt% Pd catalyst was modified by addition of copper or lead according to the protocol described in the experimental part. The results obtained on bimetallic catalysts are systematically compared to those obtained with the corresponding blank test.
For the blank test carried out in aqueous medium under hydrogen bubbling (entry 4), we notice a significant drop of the activity in comparison to that of the parent monometallic Pd catalyst. Moreover, the proportions in stearic C18:0 and trans oleic C18:1 derivatives notably increase. This loss of activity and selectivity to cis oleic isomers is probably explained by an enlargement of the metal particle size during the treatment in aqueous medium under hydrogen. This sintering phenomenon was already observed under the same conditions (hydrogen bubbling in aqueous solution) on a Pt/SiO2 catalyst [9]. The authors explained this result by the instability of the metallic particles onto the support, due to a Me–Hads binding energy that prevails over the Me–support interaction. The migration of the unstable metal particles onto the support would lead to metal growth.
The addition of copper to palladium inhibits the hydrogenating activity of palladium (entries 5 and 6), this effect being all the more obvious as the amount of copper is high. This result is in agreement with the low hydrogenating properties of copper at low temperature [1]. However, the addition of copper allows only a slight decrease in the isomerisation toward trans oleic isomers.
In the case of lead addition, the main effect observed is a significant drop of the isomerisation toward trans oleic derivatives, while the hydrogenating activity of palladium is only slightly inhibited (entries 7–9).
3.3 Addition of amines in the reaction medium
Various amounts of amines were introduced into the ethanol solvent during the hydrogenation of SOEE on the 1 wt% Pd catalyst (entries 10–23, the introduced amount of amine is given by the amine volume and the (amine/Pd) molar ratio).
The addition of methylamine and cyclic amines (piperidine, pyrrolidine, quinoline) lowers more or less the activity of the Pd monometallic catalyst. In the case of the methylamine (entry 10), for a (amine/Pd) molar ratio comparable to other entries one, the 95% conversion is not reached even for a 2-h reaction time. This phenomenon does not depend on the pKa value of the amine, since for similar (amine/Pd) molar ratios, the presence of methylamine and quinoline induces the same deactivation, in spite of pKa values equal to 10.6 and 4.9 respectively (entries 10 and 16). The three other studied amines (butylamine, triethylamine and tripopylamine), with pKa values near that of methylamine, lead to activities comparable (or even slightly higher) to that of the non-modified Pd catalyst, except in the case of butylamine for the largest introduced amount (entry 18).
The different behaviour of these two series of amine evidences a non-negligible geometric effect of amine addition in the reaction medium. In fact, the effect of amines on palladium activity can be considered as resulting of an electronic effect (positive) and a geometric one (negative). The geometric effect would prevail in the case of the first series of amine (methylamine and cyclic amines). Then, a high coverage of the catalyst surface by these modifiers, preventing the adsorption of the C18:2 molecules and their subsequent hydrogenation, can explain the observed deactivation. On the other hand, for comparable (amine/Pd) molar ratios, the electronic effect prevails for the second series of amine (butylamine, triethylamine and tripopylamine). These linear amines with different carbon chains seem thus to be able to adsorb on the surface of catalyst by leaving sufficient accessible metal sites for the hydrogenation of the linoleic molecules. The positive electronic effect is related to the electron donor character of amines, which would weaken the adsorption of the linoleic ester on palladium surface. Indeed, kinetic studies have shown a zero order for the hydrogenation of this ester, meaning a strong adsorption on the metal.
Whatever the nature of the amine, the selectivity in C18:1 is improved compared to the non-modified monometallic Pd catalyst, since the formation of stearic derivatives is inhibited. This beneficial effect of the addition of amines on the selectivity can be also explained by an electronic effect: the amines by electron donor effect would increase the electron density of the metal particles, and thus would weaken the adsorption of the C18:1 molecules on the catalyst surface. Beneficial effects of nitrogen-containing compounds addition were already observed during the selective liquid phase hydrogenation of alkynes, explained in the same way by a decomplexation or predominant ligand effect [10–13]. Moreover, the addition of amines in the reaction medium limits the cis–trans isomerisation, but also the position isomerisation of the C=C double bond of the oleic molecules after hydrogenation of the C18:2. Indeed, the distribution of the C18:1 isomers obtained for 100% conversion of the C18:2 molecules on the 1 wt%Pd catalyst shows the presence of oleic isomers with a C=C double bond in the original 9 and 12 positions, but also in 10, 11 and 13 positions (Fig. 1a). On the example displayed in Fig. 1b, the addition of triethylamine in the reaction medium ((amine/Pd)molar = 250) lowers the amount of C18:1 molecules with the C=C in 10 and 11 positions. In conclusion, the coordination of Pd with amine introduced in the reaction medium inhibits isomerisation to conjugated dienes and hence the formation of positional isomers, without affecting catalytic activity in some cases.
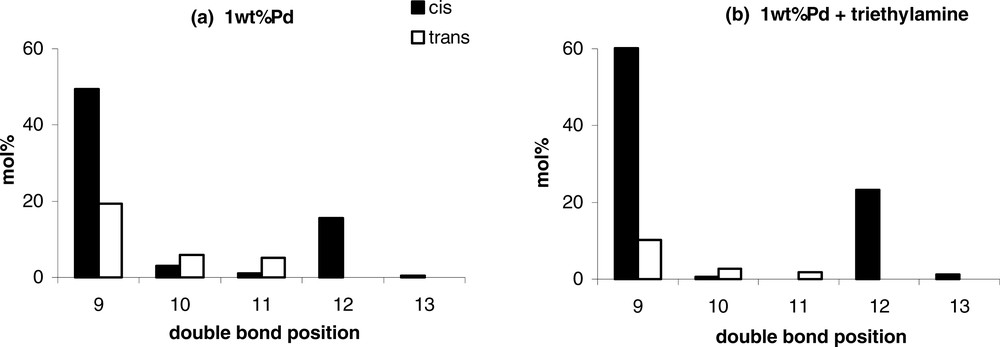
Distribution of the C18:1 isomers (position of the C=C double bond) for 100% conversion of the C18:2 molecules on: (a) 1 wt%Pd catalyst; (b) 1 wt%Pd catalyst + triethylamine ((amine/Pd)molar = 250)
4 Conclusion
From the above results concerning the hydrogenation of SOEE over silica-supported precious metals, the following conclusions can be put forward:
- • the activity decreases in the order Pd > Pt > Ru; the three metals are not selective in cis oleic isomers since trans oleic and stearic derivatives were produced;
- • the addition of copper or lead to palladium allowed us to promote the selectivity toward the cis oleic isomers, but the catalyst is less active than the monometallic palladium;
- • the best results were obtained by addition of amines in the reaction medium; in some cases, the selectivity in cis oleic derivatives is significantly improved without loss of activity.


