1 Introduction
The rich chemistry of molecules exhibiting a quinonoid structure has attracted the interest of a large scientific community for decades owing not only to their implications in organic and physical chemistry but also as ligands in coordination chemistry [1]. More specifically, the 2,5-dihydroxy-1,4-benzoquinone ligand 1 has been much used because it can provide a variety of binding sites to metal cations and has allowed the preparation of a large number of metal complexes [2]. Although the 2,5-diamino-1,4-benzoquinonediimine family C6H2(NHR)2(=NR)2, which includes 2-4, is closely related to 1, it is surprising that its coordination chemistry has remained unexplored.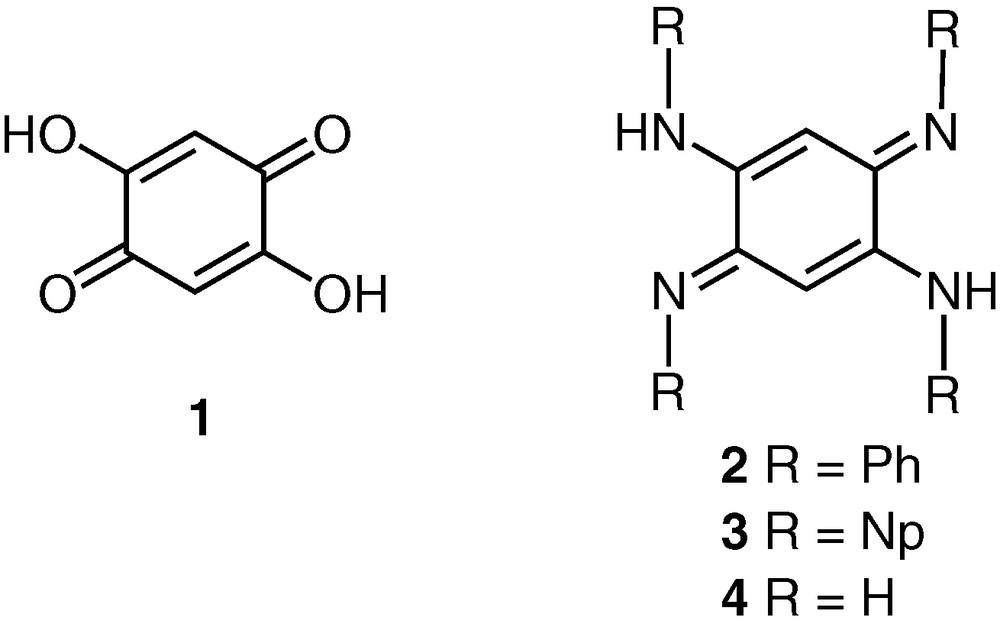
Indeed, whereas the preparation of the derivative 2 (R = phenyl), was reported more than one century ago (1875) [3], its first use as a ligand for the synthesis of metal complexes was only described in 1998 by Kaim and co-workers, who observed a para → ortho isomerisation of the quinone-diimine system induced by metal coordination and the formation of the mononuclear Cu(I) complex 5 [4]1. The stabilization of the higher-energy ortho form in 5 was suggested to result from the secondary amine functionalities in the 4,5-positions that interact with a fluorine atom of the tetrafluoroborate anion via N–HFH–N hydrogen bonding [4].


More recently, we reported the synthesis of ligand 3 in which the N-substituent is for the first time an alkyl group [5]. In its diplatinum complex 6, the π system is fully delocalised [5]. Note that Lever and co-workers recently reported the preparation of a dinuclear complex by metalation of 4 (R = H). They did not obtain the expected compound but an oxidized form of the bridging ligand, as shown in 7 [6]. Therefore, to the best of our knowledge, only two complexes have been reported in the literature, which were obtained from a metalation reaction of molecules of the types 2 and 3.
Herein, we wish to describe the synthesis of two new complexes, (8, R = neopentyl ; 10, R = benzyl), which illustrate a further aspect to be considered in the coordination chemistry of quinonoid ligands.
2 Results and discussion
Reaction of cis-[W(CO)4(NHC5H10)2] [7], which has two labile piperidine ligands, with ligand 3 [5] in THF led to the formation of a deep blue product (Fig. 1).
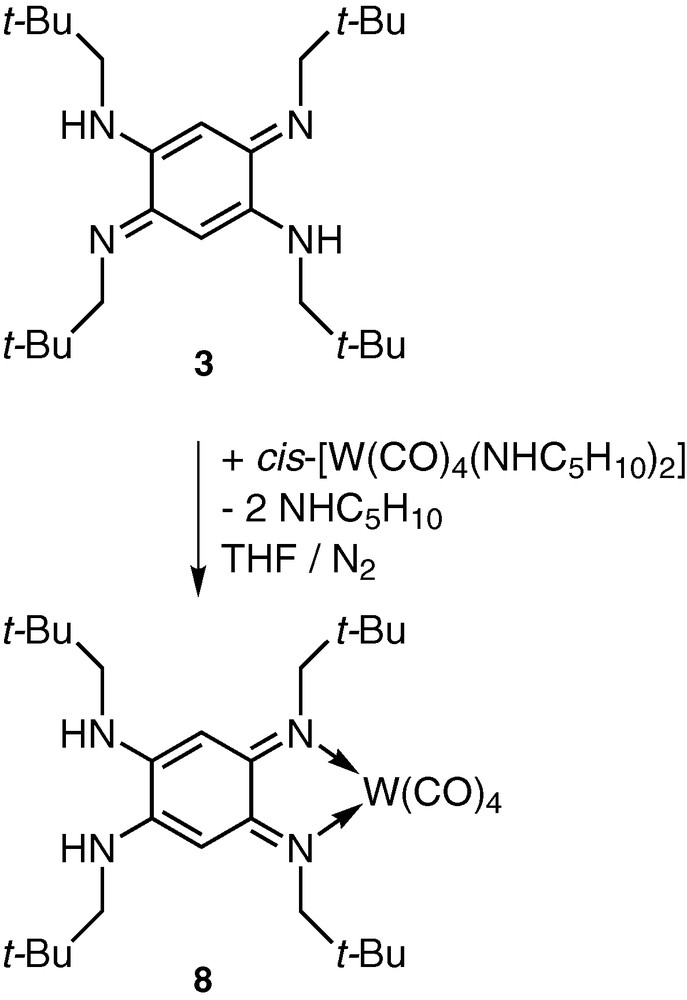
Synthesis of the mononuclear complex 8.
The spectroscopic data revealed the formation of the mononuclear tetracarbonyl complex 8, which resulted from a coordination-induced rearrangement of the electronic situation within the ligand, leading to a chelating ortho-diimine moiety. Its IR spectrum in the ν(CO) region is consistent with a cis coordination of the ligand and a C2v local symmetry about the metal centre [8]. The room-temperature 1H NMR spectra of 8 and of 3 for comparison are reported in Fig. 2 [5].

Room-temperature 1H NMR spectra of 3 (top) and 8 (bottom) in CDCl3.
Whereas the 1H NMR spectrum of 3 (Fig. 2, top) shows a structure of higher symmetry consistent with a fast intramolecular double proton transfer at room temperature involving two identical tautomers in solution [9], which generates a centrosymmetric average structure 3a (Fig. 3), the spectrum of 8 indicates the presence of two chemically different neopentyl groups, which confirms the C2v symmetry of the molecule (Fig. 2, bottom).

Intramolecular double proton transfer of 3 at room temperature.
The CH2 groups linked to the amine functions appear as a doublet at 2.81 ppm since each of them experiences a 3J(HH) coupling with a N–H proton. The CH2 groups linked to the imine functions are strongly downfield shifted (4.24 vs 2.95 ppm) upon coordination owing to the resulting decrease of electron density.
Previous studies have shown that complexes of the type [M(CO)4(α-diimine)] (M = Cr, Mo or W) play an important role in the understanding of the photophysical behaviour of chromophoric complexes with low-lying metal to ligand charge transfer (MLCT) excited states [10,11] and this prompted us to study the electronic properties of the new cis-[W(CO)4(α-diimine)] complex 8. In contrast to the UV–vis absorption spectrum of 3, which shows an intense absorption band at 340 nm that is assigned to the intraquinone transition [12], that of 8 revealed a very strong absorption at 607 nm (ε = 30 800 mol–1 dm3 cm–1), consistent with a MLCT transition (W(CO)4→N,N) [10] (Fig. 4).
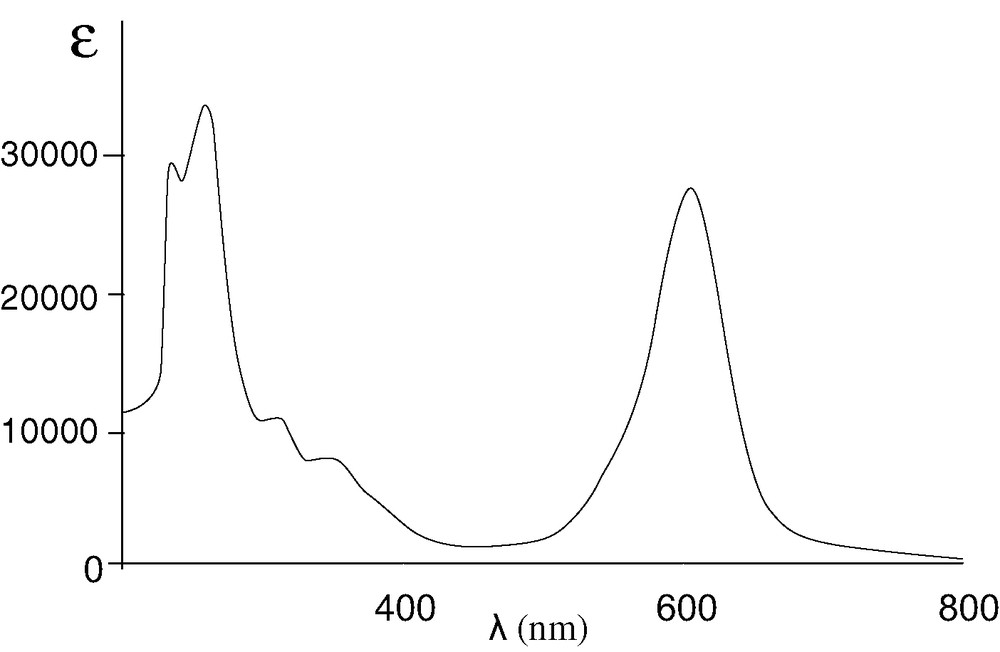
UV–visible absorption spectrum of 8 in dichloromethane (ε in mol–1 dm3 cm–1).
A fluorescence study of 8 at 345 and 606 nm in degassed CH2Cl2 revealed no significant signal at room temperature. By analogy with aryl-containing polyamine receptors, the use of benzyl groups as N-substituents is expected to lead to fluorescent probes [13–15]. Therefore, we prepared compound 9 [15] and the corresponding tungsten complex 10 according to the procedure used for the synthesis of 8.
Similarly to 8, compound 10 is deep blue and shows a similar UV-visible absorption spectrum with an intense band at 620 nm (ε = 26 500 mol–1 dm3 cm–1). No emission was observed at room temperature, although we have previously found that molecule 9 is an efficient fluorescent probe [15]. This could be explained by a transfer of energy in the excited state from the fluorescent benzyl moiety to the metal centre [16].
Although the formation of complexes 8 and 10 is related to that of compound 5, our results demonstrate that hydrogen bonding interactions involving secondary amine functionalities in the 4,5-positions are not necessary for the stabilization of the higher energy ortho form of the quinonoid structure [4]. Furthermore, Kaim et al. explained the formation of 5 by a para → ortho isomerisation induced by coordination. We prefer to describe the formation of complexes 8 and 10 as resulting from a metal-induced relocalisation of the π system of the benzoquinonediimine average structure 3a (a diimine complex related to 8 and 10 has just been obtained by tautomerization from an enamino-troponimine [17]).
3 Experimental
3.1 General
All manipulations were carried out under an atmosphere of nitrogen using standard Schlenk tube and cannula techniques. Solvents were dried and distilled under nitrogen before use. Compound 3, 9 and cis-[W(CO)4(NHC5H10)2] were prepared according to published procedures [7,12,15] and NEt3 was purchased from commercial suppliers and used without further purification. Chromatographic separations were performed on silica gel 60, 40–63 μm granulometry, Merck. The 1H and 13C NMR spectra were recorded on a AC300 Bruker spectrometer. Infrared spectra were recorded on a Perkin Elmer FT-IR 1600 spectrometer. Elemental C, H and N analyses were performed by the ‘Services de microanalyses’ (Institut Charles-Sadron, Strasbourg, France).
3.2 Synthesis of the complexes
3.2.1 Compound 8
To a yellow solution of cis-[W(CO)4(NHC5H10)2] (1.00 g, 2.15 mmol) in 150 ml of THF was added yellow solid 3 (0.90 g, 2.15 mmol) under vigorous stirring. The reaction mixture was heated to reflux. After 15 h, the green solution was filtered and the filtrate was chromatographed on a silica gel column neutralised with NEt3. First, a mixture of pentane/NEt3 (90:10, v/v) was used as eluent to eliminate all of the yellow ligand. Then CH2Cl2 was introduced to allow the migration of the blue product. Compound 8 was purified by extraction with CH2Cl2/H2O, then n-hexane was added to the organic phase and the air-sensitive product was dried in vacuo. Yield: 0.70 g (46%). Although no satisfactory elemental analyses were obtained owing to the instability of 8, the C,H,N data were consistent with a 1:1 metal/ligand ratio.
IR (CH2Cl2): ν (cm–1) = 1994 (s, νC≡O), 1891 (s, νC≡O), 1831 (m, νC≡O). 1H NMR (CDCl3): δ = 6.01 (s, 2 H, Csp2H), 4.24 (s, 4 H, CH2), 3.83 (t, 3J(HH) = 6.0 Hz, 2 H, NH), 2.81 (d, 3J(HH) = 6.0 Hz, 4 H, CH2), 1.16 (s, 18 H, CH3), 1.07 (s, 18 H, CH3). 13C NMR (CDCl3): δ = 163.17 (C=N), 141.99 (Csp2−NH), 94.52 (Csp2H), 69.50, 55.17 (CH2), 35.83, 31.33 (CMe3), 30.42, 27.86 (CH3). UV–vis (CH2Cl2, 298 K): λ (nm), (ε) (mol–1 dm3 cm–1) 259 (37400); 310 (11 700); 344 (8400); 607 (30 800).
3.2.2 Compound 10
Following a procedure similar to that described for 8, 10 was isolated as a blue solid. Yield: 17%. IR (CH2Cl2): ν (cm–1) = 1997 (vs, νC≡O), 1902 (s, νC≡O), 1837 (m, νC≡O). 1H NMR (CDCl3): δ = 7.26 (m, 20 H, Haromatic), 6.16 (s, 2 H, Csp2H), 5.44 (s, 4 H, CH2), 4.30 (t, 3J(HH) = 6.0 Hz, 2 H, NH), 4.13 (d, 3J(HH) = 6.0 Hz, 4 H, CH2). UV–vis (CH2Cl2, 298 K): λ (nm), (ε) (mol–1 dm3 cm–1) 258 (25 400); 315 (10 900) 620 (26 500).
Acknowledgements
We thank Dr Mourad Elhabiri (laboratoire de physico-chimie bioinorganique, UMR 7509 CNRS, ECPM, ULP Strasbourg) for the absorption and emission spectra. This work was supported by the CNRS and the French ‘Ministère de la Recherche et des Nouvelles Technologies’ (Paris).
1 After this manuscript was accepted for publication, a related study on Cu(I) and Re(I) complexes has been published: S. Frantz, J. Rall, I. Hartenbach, T. Shleid, S. za ́liš, W. Kain, Chem. Eur. J. 10 (2004) 149.


