1 Introduction
The bonding between asphalt and aggregate is of special importance, because it is the primary characteristic that influences the integrity of the pavement. This bonding must be established at the initial stage of the contact between asphalt and aggregate and must endure during the lifetime of the pavement. Loss of bonding results in lowered performances. DiVito and Morris [1] attribute the strength of concrete pavement to: (i) the cohesive resistance of the binder, (ii) the adhesive bond between binder and aggregate, (iii) the aggregate interlock and the frictional resistance between aggregate particles. A number of different methods have been used to strengthen the adhesion of asphalt to aggregate and to lower the pavement’s propensity to strip from the intrusion of moisture. The addition of chemical antistripping agents is determinant for such processes.
A number of research groups have studied the effectiveness of antistripping agents on the adhesion of asphalt to different types of rock surfaces. They are cationic surface-active agents, principally derived from amines. Many compositions were reported over the time. Among these are amine or quaternarized amines of omega-phenylstearic acid [2], fatty diamine/fatty acid salt and fatty amido-diamine/fatty acid salt [3,4], imidazoline derivatives [5], naphthylstearyl amine and N,N,N-trimethyl-N-phenylstearylammonium chloride [6], alkyloxyalkyleneamines and alkanol-amines [7], N-alkylpropylenediamine, 1-aminoethylimidazoline or 2-alkylimidazoline [8], norbornane amino derivatives [9,10], or long-chain amine derivatives, hydroxylalkylamines [11]. More recently, condensation products of aldehyde with organic amine, organic polyamine and hydrohalides [12], or linear polyamine with fatty acids [13] were also reported. Gilmore and Kugele [14] improved the physical properties of bitumen aggregates using a mixture of imidazoles, polyamines, alkoxylated polyamines, aminocarboxylic esters, or amides-amines, while Treybig and Chang [15] prepared antistripping additives from hydrocarbyl substituted nitrogen containing aromatic heterocyclic compounds, aldehydes, or ketones and amines. To make these materials thermally stable above 100 °C, the reactive hydrogen of the amino groups was chemically substituted with alkyl radicals, so that these materials can be stored at hot mix temperatures.
The present paper deals with the condensation of oleic acid (OA) with triethylenetetramine (TETA). Scheme 1 describes the reactions involved in this synthesis. All four products are equally possible. Since TETA is a mixture of linear and branched isomers, and of bis AEP (N,N′ -bis(2-aminoethyl)piperazine) and PEEDA (N-[(2-aminoethyl)2-aminoethyl]piperazine), the product distribution is even more complex. The condensation was carried out in the presence of beta zeolite and different solvents.
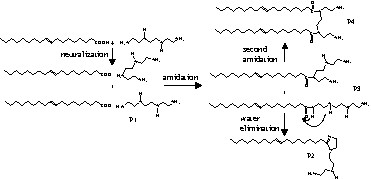
Reaction pathway of oleic acid with triethylenetetramine.
2 Experimental
The reactions were carried using OA (80%, 4:1 ratio with linoleic acid) and TETA, as substrates, and dioxane, hexane, and N,N-dimethyl-formamide as solvents. All the reactants had Merck purity. Standard catalytic tests were carried out using 20 mg of H-β zeolite H- (Si/Al ratio 25, 440 m2 g–1, from Valfor PQ), and 1.42 g of the reaction mixture (TETA:OA molar ratio of 1.05:1) dissolved in 4 ml n-hexane, dioxane or N,N-dimethyl-formamide. The catalytic tests were performed in a 10 ml stainless steel stirred autoclave or in a in a 25-ml flat bottom flask with an attached condenser dipping in a heated microsound bath. The reaction temperature varied in the range 70–190 °C and the reaction products were collected over 3.5 h. For comparison, syntheses without catalyst, and also without solvent were made as well.
The reaction products were analysed by HPLC, FTIR and UV–Vis spectroscopy, and 1H and 13C NMR. HPLC analyses were performed using an Agilent liquid chromatograph consisting of a quaternary pump, a solvent degasser, manual injection, a column oven and a multi-wavelength UV detector. Chromatographic data were acquired by means of Chemstation software (Agilent Technologies). The reaction products were analysed in the following elution conditions: Chromspher C18 column, 5 μm, 150 × 4.6, mobile phase: 10% aqueous component (with 0.3% H3PO4); 90% methanol with 0.3% H3PO4, detection: 210/500 nm. FTIR UV–Vis spectroscopy, and 1H and 13C NMR were made on DIG Lab FTS 2000 SCINTIMAR, GPS Australia Cintra 5 and Varian Gemini 300BB-300 MHz apparatus. FTIR evolution of the reaction was followed by FTIR looking for the C=O group in carboxylic acid (band at 1710 cm–1) and amides (at 1650 cm–1), and NH in amides (at 3294 cm–1).
DTBPy (ditertbutylpyridine) FT–IR measurements were performed at room temperature on a Magna-IR 550 FT-IR spectrometer from Nicolet, using a MCT-B liquid nitrogen cooled detector, and equipped with a heatable cell (up to 500 °C) with NaCl windows connected to a vacuum system and a gas manifold. Samples in the form of self-supporting pellets (around 5 mg cm–2) were placed into a carousel sample holder. Usually 200 scans were recorded at a resolution of 2 cm–1 for a single spectrum. FT–IR spectra were normalized to the weight of 10 mg cm–2. Prior to adsorption of DTBPy, the samples were dehydrated by evacuation at 400 °C, overnight. The FT–IR spectra were recorded after desorption of DTBPy at RT, 100, 150, 200 and 250 °C, respectively. The adsorption–desorption isotherms of N2 at –186 °C were obtained with a Micromeritics ASAP 2000 apparatus after outgassing the samples at 120 °C for 24 h under vacuum. These were treated using the t-plot formalism.
The resulted surfactants were tested in preparation of cationic bituminous emulsions. These have been prepared using a laboratory turbomalaxor type ATOMIX C from Emulbitume. For such purpose, penetration bitumen 80/100 was used as disperse phase and an aqueous solution of surfactant and HCl (35%) as dispersant phase. The characteristics of the resulted emulsions have been checked by determining the residual bitumen content, viscosity, breaking index of bitumen emulsion, pH and the content of particles with size between 0.16 and 0.63 mm.
3 Results and discussion
3.1 Catalytic behaviour
Tests carried out in the absence of any solvent indicated that in the interval of temperatures considered, the conversion of oleic and linoleic acid was not total, reaching a maximum of 85% at a temperature of 190 °C.
Fig. 1 shows the variation of the conversion vs reaction temperature for the reactions carried out in dioxane. Apparently, the results indicated a contradictory behaviour, with a maximum at 150 °C for reactions carried out without catalyst, and a minimum at the same temperature in the presence of the catalyst. Actually, as Fig. 2a indicates, for temperatures lower than 100 °C, it resulted mostly the neutralization of the acids in the presence of basic amines. The sum of imidazoles, primary amides, and diamides represented at most 40%. But the raise of temperature above 120 °C resulted in the formation of various amides (see Scheme 1, Fig. 2b), and the real conversion increased. Under these conditions, the concentration of the neutralized products was drastically decreased. The increase of the temperature to 170 °C led to an additional decrease of the concentration of the neutralized products. Fig. 2b also shows the effect of the catalyst. The presence of the catalyst enhances the amount of amides. If, in addition, one considers the increase of the conversion at 170 °C, one can concluded that the presence of the catalyst had a positive effect, increasing both the yield and selectivity. In the presence of the catalyst, the maximum conversion was obtained at 170 °C. The reactions carried out in hexane exhibited similar trends (Figs. 3 and 4). In the absence of the catalyst, the maximum was reached at 150 °C, while in its presence, it occurred at temperatures higher than 170 °C. In the presence of hexane, the effect of the catalyst on selectivity was even more evident. At 150 °C and in the absence of the catalyst, the concentration of the neutralization products was still very high (near 80%). In the same conditions, but in the presence of the catalyst, the concentration of these products was less than 20%. The raise of temperature to 170 °C led to the complete conversion of oleic and linoleic acids into amides.
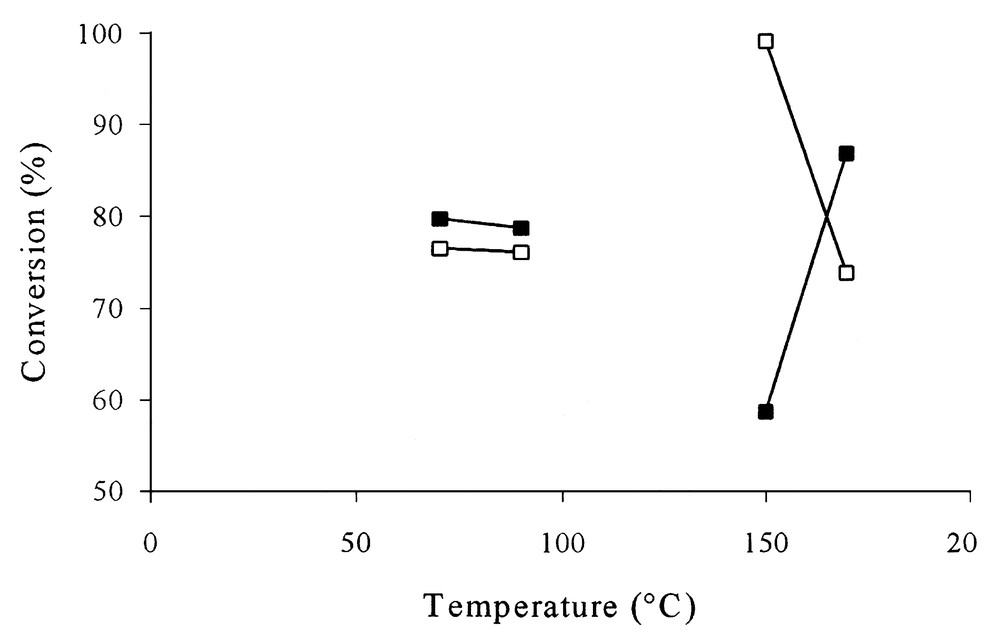
Conversion vs reaction temperature in dioxane.
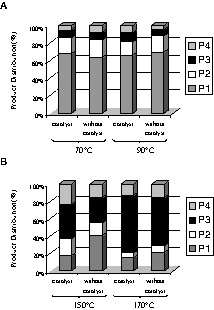
Products distribution for the reactions carried out in dioxane (P1, compounds of neutralization; P2, imidazoles; P3, primary amides; P4, diamides).
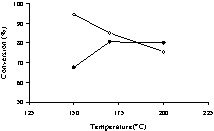
Conversion vs reaction temperature in n-hexane.
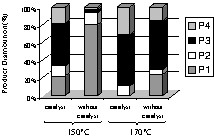
Products distribution for the reactions carried out in hexane (P1, neutralization compounds; P2, imidazoles; P3, primary amides; P4, diamides).
The reaction carried out in N,N-dimethyl-formamide occurred with high conversions in the temperature range 150–190 °C (over 95%). But the selectivity to the amides was lowered by the chemical reaction of oleic acid with the solvent.
The participation of the beta zeolite in this reaction is undoubtedly. The extent in which the surface is participating should be rather high. T-plot analysis of the adsorption isotherms of N2 indicated an external surface area of 80 m2 g–1, which means indeed a high surface. Acid sites located on internal surface can also be effective in this reaction. DTBPy-FTIR spectra contain bands at 3370, 1616, and 1530 cm–1, which can be assigned to the DTBPyH+ ion. In principle, these bands are diagnostic for Brønsted acid sites [16,17]. The complete disappearance of the OH band at 3610 cm–1 was observed, which proves the easy penetration of DTBPy into the Beta pore network. This suggests that the framework remains well accessible, even for large molecules. However, at least the acid sites located in mouth pore are expected to catalyse the reaction.
The effect of the solvent should be correlated to the hydrophobicity/hydrophilicity of the zeolite. It was already reported in literature that in acylation of naphtalene by using such catalysts a more polar solvent led to a lower conversion compared with decalin [18]. In addition to these, the use of hexane might be supported by toxicological considerations. From the reports of the Agency for Toxic Substances and Disease Registry, it results that the use of hexane seems to be more safe, although none of these solvents is perfect suitable from this point of view. While for dioxane it is still not clear whether it has carcinogenic properties, it is sure that N,N-dimethyl-formamide affects the liver and hexane the muscles.
3.2 Influence of the type of reactor
Since both the reacting molecules and the products exhibit a large tendency to agglomerate, the type of the reactor in which the amidation is carried out may have a high influence. For this reason, two different types of reactors have been used, namely, a stirred autoclave and a flat bottom flask with an attached condenser dipped in a heated microsound bath. The use of the microsound bath generally allows a better dispersion, and a desegregation of colloids. However, the comparative experiments led to very similar results (differences smaller than 5%), which might be an indication that the simple use of autoclave is effective.
3.3 Colloids formation
As mentioned above, a very important property of these surfactants is the capacity to agglomerate forming soluble colloids. DRUV-Vis spectra recorded for the reaction products indicate that the agglomeration of surfactants is a complex property depending on the reaction temperature (namely, on the conversion to amides) solvent, and catalyst. Actually, by changing these parameters, the colour of the solution changed, within the whole spectrum of colours. The data in Fig. 5 describe this relation. They clearly demonstrate the positive effect of the catalyst. As indicated above, the presence of the catalyst led to more amides, namely, to increased amounts of surfactants. The solvent also played a contribution in this process and, as shown, the use of hexane led more easily to such colloids.
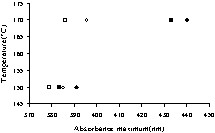
Absorbance maximum vs reaction temperature in dioxane and n-hexane (■□, reactions in dioxane; ♢♦, reactions in hexane, ■♦-catalysed reactions; □♢-reactions without catalyst).
However, it is hard to compare the catalytic behaviour of the beta zeolite with other heterogeneous catalysts because, to our best knowledge, this is the first heterogeneous by catalysed amidation of fatty acids with amines. Other catalytic reactions of these acids only considered enzymatic catalysis using lipases [19,20]. Generally, these enzymes behave as acidic catalysts. In the presence of the inorganic beta zeolite, one may suppose that the amine is chemisorbed on the acidic sites favouring the reaction with fatty acid. The role of the solvent is to extract the resulted amide and dissolve it, leading to stable colloidal solutions.
3.4 Surfactant properties
Table 1 compiles the characteristics of emulsions prepared using surfactants prepared in hexane, dioxane and DMF. It is worth noting that all the emulsions were stable, irrespective of the way in which the surfactants were synthesized. However, the characteristics of the prepared emulsions are in a perfect concordance with the content of amides in the batches. Granulometric analysis (from the content in particles with size higher than 0.63 mm, and the content in particles with size between 0.16 and 0.63 mm) indicates that the emulsions obtained using samples prepared in hexane are superior to those obtained from samples prepared in dioxane or DMF.
Characteristics of emulsions prepared using surfactants prepared in hexane, dioxane and DMF
| Characteristics | Surfactant prepared in | ||
| hexane | dioxane | DMF | |
| Content in residual bitumen, % | 60.1 | 60.3 | 60.9 |
| Viscosity, °E | 5.1 | 4.9 | 5.4 |
| Breaking index of bitumen emulsion, g filler Sikaisol/100 g emulsion | 52 | 57 | 48 |
| pH | 3.1 | 3.0 | 3.2 |
| Content in particles with size higher than 0.63 mm, % | 0 | 0.2 | 0.71 |
| Content in particles with size between 0.16 and 0.63 mm, % | 0.12 | 0.24 | 0.95 |
4 Conclusion
Synthesis of amido-type surfactants occurs with very good conversion and selectivity in the presence of beta-zeolite as catalyst. The reaction carried out in several solvents indicated hexane as the most effective. The surfactants obtained under these conditions led to very stable emulsions. However, the surfactants prepared in hexane led to better characteristics of these emulsions.


