1 Introduction
Chemical fertilizers used in conventional agriculture are well known to pollute the environment, particularly the ground water. Entrapping, encapsulating or dispersing the fertilizer [1] in a polymeric matrix could be a good way to minimize these effects. Using a biodegradable polymer can allow a controlled release of the fertilizers avoiding additional residue in the soil.
Poly(ε-caprolactone) (PCL) is especially desirable for encapsulating active agents because it is biodegradable and is available at low cost. It was already used to encapsulate many water-soluble [2] and water-insoluble [3–5] active agents. Generally, the encapsulation results from different preparation methods based on emulsification processes [2–10], but this way is rather used for drug release.
The aim of our work is the development of new composite materials from biodegradable polyester and wood (shaving, sawdust, flavour…) that can act as carriers of active agents. The active agent used is the potassium chloride (source of potassium chloride in agriculture), it was incorporated in the release system that consisted of PCL and pine. The originality of this system is the use of wood, which can bring to the system a hydrophilic behaviour and porosity; moreover, this new system allows the valorisation of wood waste.
In a first time, the influence of each constituent (wood, potassium chloride and water) of the system on the polymerisation of ε-caprolactone (ε-CL) was evaluated.
Depending on the wood shaped, two kinds of systems were studied. The first one consists in impregnating porous wood samples with the active agent [11–14], followed by the impregnation of ε-caprolactone, which is polymerised inside the wood. PCL is supposed to fill the micro-channels of porous wood. On the other hand, it is possible to carry out materials as mixtures by including flours of wood and potassium chloride in a matrix of poly(ε-caprolactone).
The release of KCl for these two systems was studied.
2 Experimental
2.1 Materials
ε-caprolactone (ε-CL), stannous-2-ethylhexanoate (Sn(Oct)2) (Acros and Sigma-Aldrich, Saint-Quentin-Fallavier, France), methanol, dichloromethane (Carlo Erba) were used as received. Potassium chloride (KCl) (Acros) was crushed (from 160- to 310-μm diameter), sieved and dried at 130 °C in an oven. Pine was ultra dried at 250 °C, crushed (from 160- to 310-μm diameter), sieved and kept at 130 °C. Ultra-pure water (Milli Q Plus, Millipore, Molsheim, France) was used to study the release of KCl.
1H NMR spectroscopy was performed on a Bruker AC 250 spectrometer. Tetramethylsilane was used as an internal standard. Size Exclusion Chromatography (SEC) analyses were performed with a Waters 410 permeation chromatograph equipped with two Waters Styragel columns (HR 0.5 and HR 4E) at 37 °C and a refractometer, using THF as the eluent (1.0 ml.min–1), polystyrene as calibration standards, and Waters Millennium 3.05 as data processing software. Titration of KCl released was made using a Schott Geräte titration unit (Titroline alpha). Blend systems were made with a Bradender PL2000 Plasticorder internal mixer.
2.2 Polymerisation of ε-caprolactone
Typically, a mixture of 20 ml of ε-caprolactone (1 eq.) and 584 μl of stannous octoate (Sn(Oct)2) was introduced in a reactor equipped with a mechanical stirrer. Depending on the studied parameters, different amounts of water (from 0 to 0.1 eq.), or wood (from 0.1 to 19%) or KCl (from 0 to 1 eq.) were added to the solution of monomer. In all the cases, the polymerisation took place at 100 °C during 24 h.
2.3 Preparation of impregnated wood samples
Pine was ultra-dried at 250 °C. The wood samples were first cut into pieces of 1 × 1 × 2-cm3 dimensions and all pieces were brought to constant weight (m) in a vacuum at room temperature for one hour. There after, the impregnating KCl solution (3 mol.l–1) admitted under vacuum until all wood samples were covered for 2 h. The samples were dried at 60 °C during 24 h and weighted (M1 = 1.4 m). At this time, the resulting systems are only consisted of wood and potassium chloride (Wood-KCl). Titration of KCl extracted from different parts of wood samples (extremities, middle and three layers) shows that the fertilizer is well dispersed in all the volume.
To prepare wood–KCl–PCL system, wood–KCl samples were placed under vacuum during 1 h. The mixture of ε-caprolactone and stannous octoate (first heated at 60 °C) was admitted under vacuum until all wood samples were covered for two hours. At the end of this period, the vacuum was released. The impregnated samples were covered by hot sand (130 °C) for 17 h to allow the polymerisation and to prevent the impregnated monomer from evaporating. Weighted samples (M2 = 1.9 M1) was analysed to show that the polymerisation occurred in all the volume. The resulting system was composed of wood, KCl and poly(ε-caprolactone) (wood–KCl–PCL) (
2.4 Preparation of blends wood–PCL–KCl
In a reactor equipped with a mechanical stirrer, 1 equivalent of ε-CL and 0.01 equivalent of stannous 2-ethylhexanoate were mixed and heated at 120 °C. The reaction was carried on for three hours. Then the polymer in dichloromethane was precipitated in methanol and was dried under reduced pressure. At this stage,
Composite was obtained by mixing poly(ε-caprolactone), wood (flavour) and KCl at 80 °C for 20 min (20 rpm) with an internal mixer. Small paving stones of composite were implemented by pressing material between two metal plates (1.5-mm thickness) at 130 °C. To obtain the wanted molar masses, the polymerisation was conducted with different amount of water: with no water, the obtained masses were
2.5 Study of the release
Impregnated wood samples (1 × 1 × 2 cm3) with KCl and/or PCL were placed in 500 ml of ultra-pure water at pH = 6. At given time intervals, 5 ml were removed and titrated with nitrate silver (0.02 mol.l–1).
Blended samples (50 mg, 5 × 5 × 1.5 mm3) were prepared and placed in 500 ml of ultra-pure water at different pH (5 or 9). At given time intervals, 5 ml was removed and titrated with nitrate silver (0.02 mol.l–1).
3 Results and discussion
3.1 Polymerisation of ε-caprolactone
Ultra-dried wood contains about 1–2% of water. The influence of water on polymerisation of ε-caprolactone was studied. Polymerisation rate is determinate by 1H NMR spectroscopy from the relative intensity of the ester methylene (–C(O)OCH2–; δ = 4,06 ppm) of PCL and the ester methylene of ε-CL (–C(O)OCH2–; δ = 4,24 ppm). The curves showed that the rate of polymerisation for sample containing 0.01 equiv of water increased over 2 times in comparison with the polymerisation conducted without water (Fig. 1a and b). On the other hand for higher water amounts (0.1 eq.), the polymerisation rate decreased over 5 times. In addition, the decrease of the molar mass with the increase of water amount showed the influence of water as an additional initiator.
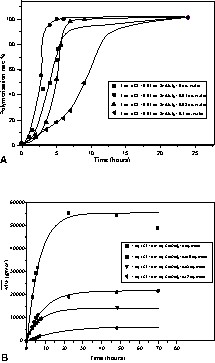
(A) Conversion data for the polymerisation of ε-caprolactone in which various quantities of water were added. (B) SEC analysis for polymerisation conducted with various quantities of water.
These results were in accordance with the works of Kovalsky [15] and Storey [16] that have studied polymerisation of ε-caprolactone in the presence of alcohols (ROH=BuOH) or water. According to their conclusions, in the presence of an alcohol (ROH), if the ratio [ROH]0/[Sn(Oct)2]0 does not exceed approximately 2, ROH acts mostly as a co-initiator, reacting with Sn(Oct)2. Above this ratio, ROH becomes additionally and predominantly a chain transfer agent. These conclusions could be transposed to the results obtained with water. When the ratio [water]/[initiator] was less than 2 (polymerisation conducted with 0.01 eq. of water), the conversion time of ε-CL was faster than for the polymerisation conducted without water.
SEC analysis showed that even if the conversion of ε-caprolactone was achieved after 8 h, the molar masses carried on increasing for 15–20 h. This indicated participation of condensation polymerisation whereby water-initiated chains bearing a carboxylic acid head group and hydroxyl tail group acted as A–B monomers.
In addition, the influence of the wood and KCl on polymerisation was evaluated. Indeed wood is an heterogeneous material containing molecules (phenol, lignin…) that could interfere on polymerisation. The polymerisation of ε-caprolactone was studied with different amounts of wood (0.1% to 19%).
The obtained results showed that the polymerisation rate decreased with the increase of the wood amount but after 8 h of reaction, the conversion is achieved in all cases.
Nevertheless, wood amounts have an important influence on the molar masses of PCL: these latter decreased with the increase of the amount of wood:
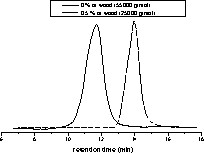
SEC analysis of poly(ε-CL) for polymerisation conducted with or without wood.
This result could be explained by the presence of residual water and alcohol molecules in the ultra-dried wood [15,16].
Concerning KCl amount, it has a big influence on molar masses of PCL, because KCl is well known to be a hygroscopic compound, and water is known to slow down the polymerisation.
3.2 Release of KCl
The release of KCl was studied on two kinds of systems:
- • the wood samples impregnated with KCl and/or PCL;
- • the blends PCL–wood–KCl
Concerning the wood impregnated samples, the release of KCl was conducted on samples impregnated with PCL or not (
In the case of blends wood–KCl–PCL [17], different parameters were studied to evaluate their influence on the release of KCl: the pH of the water, the molar masses of PCL and the quantities of wood. Fig. 3 shows the time course of KCl release from blends containing different amounts of wood (1% or 11%), where PCL has different molar masses (
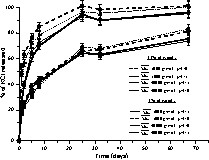
Time course of potassium chloride release from different blends wood–PCL–KCl containing 1 or 11% of wood, pH = 5 or 9,
The release rate of KCl from composite was in all cases initially fast but slowed after 5 days. Two behaviours can be distinguished: samples having 1% wood content, and samples having 11% wood content. This proves that the main influence on the release is the wood amount. Indeed, KCl release from blends having 11% wood content is more important than from blends having 1% wood content: wood brought porosity to the material, the micro-channels throughout the material allowed an easier diffusion of KCl. Increases in amount of wood produced a corresponding increase in drug release.
The influence of pH was very small. The release rate of KCl from blends seems to decrease with an increase in molar mass of PCL. For example, for samples having 1% of wood, at pH = 5, the release rate of KCl from samples with
4 Conclusion
This study showed that the release of KCl in water from blends of PCL/wood could be increased by the increase of the wood amount into the PCL matrix. Concerning the release studies of impregnated wood samples, the wood amount is so high that the release of KCl is very fast. About the polymerisation of ε-CL in this kind of system, the three parameters (water-wood-KCl) mainly decrease the molar masses obtained.
In conclusion, the release of KCl could be controlled for blends PCL/wood by varying the amount of wood.
Acknowledgements
This work has been performed within the ‘Réseau matériaux polymères–plasturgie’ interregional network (‘Pôle universitaire de chimie organique’). We gratefully acknowledge financial support from the ‘Ministère de la Recherche et des Nouvelles Technologies’, CNRS (‘Centre national de la recherche scientifique’), the ‘Région Basse-Normandie’ and the European Union (FEDER funding) for financial support.


