1 Importance of surfactants: market and uses
Surfactants constitute an important class of chemical products, not only because they are commonly used but also because they have a great variety of applications (Tables 1 and 2) in households, industry and agriculture [1–3]. End users encounter surfactants in detergents, cosmetics and food, in manufacturing processes, surfactants are used as anti-static agents, lubricants and levelling agents (e.g., for textile production), de-inking of recycled paper, and as emulsifiers (in the food industry and for the production of dyes, coatings and plastics).
Major sectors of surfactants use (in millions of tons) [1]
| Household detergents | ±4 |
| Personal care | ±0.9 |
| Textile | ±0.7 |
| Oilfield | ±0.4 |
| Industrial cleaning | ±0.5 |
| Crop protection, agrochemical formulation | ±0.2 |
| Emulsion polymerisation | ±0.3 |
| Food | ±0.2 |
| Construction | ±0.5 |
| Pulp and paper | ±0.1 |
| Plastics | ±0.1 |
| Leather | ±0.1 |
Functional properties of surfactants [1]
| Detergency |
| O/W emulsifier |
| W/O emulsifier |
| Foaming agent |
| Solubilizer and disperser |
| Crystal growth inhibitor (oil, ice) |
| Lubricant |
| Starch retrogradation inhibitor |
| Anti foaming agent |
| Wetting agent |
| Rheology modifier |
| Inhibition of hydrates |
Worldwide production of surfactants amounted to 17–19 Mt in 2000 (including soap for less than 50%) [2,3]. The expected future growth rates is 3–4% per year globally and 1.5–2.0% in the European Union. The growth rate is closely related to the world demand in detergents, since this sector uses over 50% of surfactant production [4].
Being an important group of products of the chemical industry, synthetic surfactants play a significant role in economic and socio-economic terms. Their production and use implies environmental and health impacts. Consequently, industry and research organisations are currently active in finding out new ways of producing surfactants which are more environmentally friendly and safer, which entail a minimum health risk and which can be produced from domestic renewable resources (corn, beet...) [1,2]. The introduction of more sophisticated, high performance products can also help to reduce greenhouse gas emissions [4,5]. For example, the new generation of detergents wash effectively at much lower temperatures, resulting in significant energy saving [3].
2 Surfactant raw materials
Surfactants can be derived from both petrochemical feedstock and renewable resources (plant and animal oils, micro-organisms). They were originally made from renewable resources like fats and oils, whereas today, the majority is of petrochemical origin [3]. However, renewable resources play an important role in the market since they account for about one third of the total organic carbon fixed in surfactants, with two thirds of the organic carbon being of fossil origin.
Among renewable raw materials, oleochemical products represent half of the total surfactant production.
Given the total surfactant production of 2.5 million tonnes in 2002 (excluding soap), the use of renewable raw materials (RRM) for surfactant production currently exceeds by far the amount used for all other purposes. Among these purposes, one can point out polymers, lubricants and solvents. The estimated EU potential of RRM-based products in 2010 is 500 000 tonnes for polymers, 200 0000 tonnes from lubricants, 235 000 tonnes for solvents and 1 180 000 tonnes for surfactants with the highest share of 50% compared to the total consumption market [3].
A surfactant can be categorised as anionic (negatively charged), cationic (positively charged), non-ionic (no charge) and zwitterionic (containing both a positive and a negative group on a large pH scale). Anionics and non-ionics represent the largest groups of surfactants (Fig. 1). They include both products based on petrochemical and oleochemical raw materials. Cationics (mainly used for fabric softening) and amphoterics (e.g. used for bodycare preparations) have been restricted to comparatively small markets so far [6].
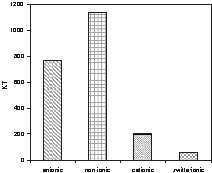
Captive use (year 2005) of surfactants by ionic type in western Europe.
Nearly all renewable raw materials currently used for surfactant production in Europe are tropical vegetable oils. Their chemical structure is more suitable than oils produced from European crops such as sunflower and oilseed rape. Starch-based surfactants [7], especially alkylpolyglycosides (APG) represent an important exception but they currently account for less than 3% of total production [4]. The proportion of oleochemical to petrochemical-based surfactants in the market is very sensitive to price.
Current regulations do not give any competitive advantage for oleochemical or biosurfactants from microorganisms versus petrochemical surfactants. However, additional policies and measures could increase the market share of oleochemical surfactants or biosurfactants (without taking into account biosurfactant with special biological activities and very high added value) above 40% by 2010. A number of the politic measures would help in this respect.
As one of its activities within the European Climate Change Programme (ECCP), which ran from March 2000 to June 2001, the European Commission assessed the potential to mitigate greenhouse gas emissions by increasing the use of renewable raw materials as a chemical feedstock [3]. Surfactants were identified as one of the product groups that – if produced from biogeneous feedstock in larger amounts – can contribute to the reduction of greenhouse gas emissions. For the assessment prepared under the ECCP the increased use of tropical vegetable oils (coconut oil, palm oil...) was assumed. Further substantial greenhouse gas emission reduction could be achieved by innovative surfactants based on domestic crops or produced by microorganisms.
3 Research on new surfactants
Numerous researches have been focussed Š on the screening of new surfactants in recent years. The reasons are not the improvement of technical properties because the main classes of traditional surfactants such as ethoxylates, alkyl benzene sulfonates, alcohol ether sulphates, and alcohol sulphates are performant. Two new strategic approaches are actually taken into account. The first one results from the impact on environment. Traditional surfactants exhibit a low rate of biodegradation and a high potential aquatic toxicity.
For these reasons, new green surfactants are promising even if their performances could be slightly inferior or their price more expensive. Among these surfactants, alkylpolyglycosides are the most successful at this time [8].
The second strategic approach is to develop new functionalities for surface-active molecules. It could be technofunctionalities like surface activity and polymerizability combining in one molecule. It could also be due to new and interesting properties such as gemini [9], dimeric surfactants made up of two identical amphiphilic moieties or surfactants possessing biological activities (see § 4) [10,11].
4 Green surfactants, biosurfactants
Interest in biosurfactants has increased considerably in recent years, as they are potential candidates for many commercial applications.
The term biosurfactant has been used very loosely and refers to any usable and isolable compound obtained from microorganisms that has some effects on interfaces. It is important to notice that other biosurfactants can be extracted from biomass or obtained from it after biotransformation.
Applications in many areas are possible for example in agriculture, in detergency, in public health, in food petroleum extraction, in waste utilisation, in bioremediation, in environmental pollution control [12–14]…
A large variety of microorganisms are known to produce biosurfactants, which vary in their chemical properties and molecular size (see Table 3 for examples). However, up to now, biosurfactants compete with difficulty against the chemically synthesized compounds on the surfactant market, due to their high production costs (at least 50 times more expensive, depending of the biosurfactant and its purity).
Main types of biosurfactants produced by microorganisms [15,18,19]
| Main types | Examples | Microorganisms |
| Glycolipids | Trehalose lipid | Rhodococcus erithropolis |
| Sophorose lipid | Torulopsis magnoliae, Candida bombicola | |
| Rhamnose lipid | Pseudomonas aeruginosa | |
| Mannosylerithritol lipid | Shizonella melanogramma, Pseudozyma Antarctica | |
| Liposaccharides | Emulsane | Acinetobacter calcoaceticus |
| Alasan | Acinetobacter radioresistens | |
| Lipopeptides | Surfactin | Bacillus subtilis |
| Viscosine | Pseudomonas fluorescens | |
| Phospholipids | Corynebacterium lepus, Aspergillus | |
| Fatty acids and neutral lipids | Corynomycolic acid | Corynebacterium insidibasseosum |
It is thus very important to develop strategies in order to facilitate their industrial development:
- • by a reduction of the production costs (micro organisms selection, genomic, metabolic engineering, fermentation, purification procedures…);
- • by developing alternative routes of production (bio transformation of natural products);
- • by a convenient valorisation of their specific properties.
Biosurfactants have special advantages over their chemically manufactured counterparts because of their lower toxicity, their biodegradable nature and effectiveness at extreme temperature, pH and salinity for several of them. In addition, they possess surface-active properties differing in many cases from synthetic surfactants [15].
Most of their applications depend on their properties at a nano-scale level and their ability to self-assemble.
In addition, several biosurfactants have been reported to have manifold biological activities covering antibiotics, fungicidal, insecticide, antiviral and antitumoral agents, immunomodulators or specific toxins and enzyme inhibitors [15,16]. In this way, they receive increasing attention for therapeutic applications. Developing knowledge of these biological properties is a key factor for introducing these molecules in high-added value products and industries.
Though the mechanism of action of the majority of such compounds has not been clarified in detail so far, it is obvious that their surface and membrane-active properties play an important role in the expression of their activities.
Another striking advantage of biosurfactants over chemically synthetic compounds is the possibility to modify them (by genetic engineering, biological or biochemical techniques) in order to meet specific requirements [17].
Microbes are major candidates in the search for enlarging our present range of biosurfactants. A lot of them produce a complex mixture of biosurfactants, particularly during their growth on water-immiscible substrates. Among microbes, and until now, a majority of biosurfactants is found to be produced by bacteria (see Table 3 for examples). Nevertheless, yeast (e.,g., Torulopsis) and especially fungi remain an important stock of valuable molecules that have been the subject of very little screening.
Generally, biosurfactants are metabolites with the typical amphiphilic structure where the hydrophobic moiety is either a long-chain fatty acid, an hydroxy fatty acid or an α-alkyl-β-hydroxy fatty acid. The hydrophilic moiety is a carbohydrate, one several amino acids, a cyclic peptide, a carboxylic acid, an alcohol…
Fig. 2 illustrates the chemical structure of a fengycin and a rhamnolipid as example of lipopeptides and glycolipids, respectively. Homologous and isoform molecules are often produced by the same microorganism.
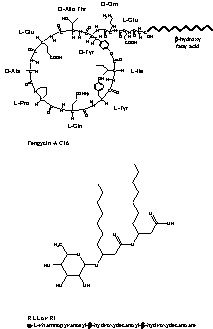
Structure of a fengycin and a rhamnolipid.
Physical and chemical properties, surface tension reduction, foam capacity and stability, emulsion capacity and stability, low critical micellar concentrations, solubility, detergency power, wetting ability are very important in the search of a potential biosurfactant. These properties are used in the evaluation step of their performance and in the screening operations of potential microorganisms for biosurfactants production. Many of the structures and properties of biosurfactants differ from synthetic ones, providing new possibilities for industrial applications. Nowadays, the major classes of biosurfactants include glycolipids and lipopeptides. Others are phospholipids and fatty acids, polymeric surfactants and particulate surfactants (see Table 3).
Considerable attention must be paid to the production of biosurfactants via biotransformations for several reasons:
- • it exists a close structural similarity between some groups of commercial surfactants and biosurfactants (e.g.: sucrose esters and glycolipids);
- • it is possible to produce commercial biosurfactants from biomass;
- • it is possible to derive different hydrophilic and hydrophobic moieties through microbial fermentation or enzymatic treatments.
Moreover, the use of hemi synthesis can significantly enlarge the potential for obtaining new molecules and finding better formulations for specific applications.
5 Need of a multidisciplinary approach
The future of biosurfactants and surfactants from renewable materials will be governed by the net economic gain between the production cost and application benefit. Moreover the links between the production parameters of these molecules, their structure and their functions is a clear necessity to optimise the strategic view of their industrial development.
The production of biosurfactants suitable for specific applications and the development of high added-value properties is the central part of the future research development project (Fig. 3), reinforced by structured screenings and computational modelisation.

Pathways for biosurfactants production and applications.
Table 4 illustrates a horizontal approach allowing us to find the best molecules for a given property or for multifunctional properties. This step can be applied for example on lipopeptides varying in their hydrophobic moieties. Analogous molecules can also be synthesized in order to improve this approach.
Horizontal approach allowing selection of the most efficient lipopeptides (for example)
| Physico-chemical properties | ↔ | Structure-function relationships | ↔ | Biological activities |
| Solubility | Monolayer coverage | Antibiotic | ||
| Critical micellar concentration | Molecular orientation | Fungicide | ||
| Surface tensions | Surface bonding | Insecticide | ||
| Foaming capacity, emulsion capacity and stability | Liposome permeability | Antiviral | ||
| Adhesion | Dynamic surface properties | Immunomodulator | ||
| Cleaning efficiency | Titled peptide inactivation | Toxicity | ||
| Haemolysis |
Once the most efficient lipopeptide is selected, a vertical approach is necessary to select the best way of production, the purity level of the product in relation with the field of application, the purification procedure, the scale-up parameters...
It is obvious that at each step of the channel, a horizontal approach is also a necessity. Indeed it is very useful to have the multi sectorial view and to integrate several disciplines and technologies (modelling, nanotechniques and nanotechnologies, captors, biotechnology...).


