1 Introduction
Since the earliest spin-crossover (SCO) complexes were presented by Baker and Bobonich in 1964 [1], almost every characterisation technique has been employed in an effort to understand comprehensively the nature of these materials. In brief, SCO is a phenomenon that occurs where the energy difference between the high-spin state and the low-spin state for a given metal is ~kB T (where T is the temperature and kB is the Boltzmann constant). When this criterion is fulfilled, switching between spin states on a molecular level can be stimulated using changes in temperature or pressure, and in some cases by irradiation with light. This bistability has led to an increasing interest in using these materials in technological devices.
By far the most widely studied examples of this behaviour concern iron(II) octahedral complexes, but all first row transition metals with electronic configuration d4 to d7 have been shown to exhibit SCO behaviour [2,3]. This is not a phenomenon limited to octahedral complexes, however, and a report has recently been published concerning the structural changes associated with SCO, in an unusual tetrahedral cobalt complex [4]. Iron(II) compounds have the electronic configuration d6, which can exist in a paramagnetic, 5T2 high-spin state (t2g4eg2) or a diamagnetic, 1A1 low-spin state (t2g6) as shown in Fig. 1. In general, a transition from high to low-spin takes place on cooling or increasing pressure, a change that is associated with a contraction in iron-ligand distance (typically ~0.2 Å for nitrogen donor ligands) and a regularisation of the octahedral geometry. These structural changes are best reflected by comparisons of the volume of the FeN6 octahedron, which is typically about 13 Å3 for the high-spin state and approximately 10 Å3 for the low-spin state.

SCO in iron(II) d6 from the paramagnetic, 5T2, high-spin state (t2g4eg2) to the diamagnetic, 1A1, low-spin state (t2g6).
In some cases, it is possible to convert the low-spin ground state into a metastable high-spin state with a prolonged lifetime using irradiation with light at low temperature. This effect is known as Light Induced Excited Spin State Trapping (LIESST) [5] and the limiting temperature at which the trapped high-spin state is lost is known as TLIESST [6].
SCO has been reported widely in both the solid and liquid state. In the liquid state the transitions are gradual following the prediction of the Boltzmann distribution between two vibronic manifolds. In the solid state, the transitions generally become abrupt due to cooperative interactions between iron centres that communicate through weak intermolecular interactions. Increasing the cooperativity within SCO compounds leads to sharp transitions and, in more extreme cases, promotes hysteresis, both of which are vital if the complexes are to have any real technological impact. In addition, it is important that the transition takes place as close as possible to room temperature, where a simple Peltier element can be used to induce the transition [7,8]. Since the change in spin state leads to changes in magnetic, optical and structural properties there has been considerable interest in the potential of SCO complexes as molecular switches, magnetic storage devices, laser displays and more recently, intelligent contrast agents for Magnetic Resonance Imaging [9]. For these ideals to be realised, however, cooperativity is vital and our detailed understanding of the behaviour of these fascinating materials at a molecular level must be increased. Our own interest in these materials is primarily structural, with the object of correlating the physical properties with structural changes and characteristics. To this end, our initial work focussed primarily on two families of iron(II) complexes: bis(thiocyanato) derivatives containing ligands with highly delocalised π electron systems, e.g. [Fe(phen)2(NCS)2] (1) and [Fe(PM-BiA)2NCS)2] (2) (where phen = 1,10-phenanthroline and PM-BiA = N-2′-pyridylmethylene 4-aminobiphenyl), and derivatives of [Fe(bpp1)2](X)2 (where bpp1 = 2,6-di(pyrazol-1-yl)pyridine and X is an anion, usually ClO4– or BF4–). We will discuss the most interesting aspects of our work on these materials before progressing to a description of structural work carried out on [di(hydro)bis(1-pyrazolyl)borate] iron complexes and some novel SCO coordination polymers.
2 Bis(thiocyanato) Fe(II) complexes
From magnetic susceptibility measurements, König and Madeja [10] found that [Fe(phen)2(NCS)2] (1) undergoes an abrupt transition at 176 K and the high and low-spin state structures were determined at ambient temperature and 130 K, respectively, by Gallois et al. [11], where the compound was found to be orthorhombic (Pbcn) over the whole temperature range. Decurtins et al. demonstrated that complete conversion to the metastable high-spin state is possible under irradiation with laser light, λ = 647 nm [12,13].
In 2002 we reported the crystal structure determination of the low-spin state at 30 K, together with that of the metastable high-spin state after irradiation at 30 K [14] (Fig. 2). Prior to this, structural information about the metastable state had been limited to that obtained from EXAFS studies [15]. On irradiation, there is no change in space group and the overall packing is the same as that seen in the room temperature, high-spin state. The main intermolecular interactions are π–π interactions between aromatic rings of the ligands of neighbouring molecules and hydrogen bonds between the aromatic C–H groups and the sulphur atoms of the thiocyanate ligands. The iron–nitrogen bonds and intermolecular distances are shorter in the metastable high-spin state, than those in the high-spin state at room temperature. This suggests that the metastable high-spin state has a more cooperative crystal network than the high-spin ground state at ambient temperature. This result is important due to the critical role that the cooperativity is believed to play in determining the course of the spin transition. The knowledge of the structure of the compound in the high-spin state at low temperature, allowed direct comparison with that of the low-spin state at the same temperature thus making possible a direct determination of the effect of the spin transition on the structure, divorced from the temperature effects associated with the thermal spin transition.

Overlay of the low-spin and metastable high-spin states of [Fe(phen)2(NCS)2] (1) at 30 K. The average Fe–N distance increases from 1.983(5) to 2.122(5) Å and the octahedron becomes more irregular on excitation.
Further to this, we have studied extensively, several related materials with the aim of developing systems that exhibit strong cooperativity and hence show abrupt transitions with large hysteresis. This has been carried out using ligand substitution, primarily the replacement of the phenanthroline with for example, PM-BiA (where PM-BiA = N-2′-pyridylmethylene 4-aminobiphenyl, Scheme 1) [16]. [Fe(PM-BiA)2NCS)2] (2) was studied in both the high and low-spin states (both orthorhombic, Pccn) and in 1998 the existence of a second, monoclinic polymorph (2-II) was confirmed [17,18]. The orthorhombic polymorph, 2-I, has an extremely abrupt spin transition centred at 167 K with a hysteresis loop of 5 K. 2-II, by contrast, shows a more gradual transition centred at 198 K, with no hysteresis. 2-I also undergoes LIESST at low temperature (TLIESST = 78 K [17]), but attempts to determine the structure of this material in the metastable high-spin state at low temperature after irradiation, have hitherto proved unsuccessful. However, recently we have shown that it is possible to trap the high-spin state of 2-I by flash freezing the crystal to 30 K in a flow of chilled helium gas [19]. This is a new way to introduce bistability into a SCO material and has been investigated by X-ray diffraction.

Selected bidentate ligands.
After flash freezing the crystal of 2-I there is a difference in the unit cell volumes between the trapped high-spin state and the low-spin state (ΔVSC) at 30 K of 160 Å3; 4.5% of the room temperature unit cell volume. Relaxation times from the trapped high-spin state were found to be very long, and flash cooling to any temperature below 80 K, results in trapping of the high-spin state, for a few hours at least. Above 80 K, relaxation is much faster and trapping of the metastable state is not possible. Results obtained at 80 K show that flash cooling to this temperature traps the metastable state for a few minutes before relaxation back to the low-spin state, as shown by monitoring the unit cell parameters. It is interesting to note that this temperature limit for the retention of the high-spin state by flash cooling is close to TLIESST (78 K). Comparison of the octahedral volumes and the octahedral distortions shows that 100% of the molecules were trapped in the high-spin state by flash freezing to 30 K.
Although there is no change in space group either on cooling slowly or flash freezing, significant structural differences were noted between the high-spin state at room temperature and that resulting from flash freezing. The magnitude of the π–π interactions is smaller in the trapped high-spin crystal structure than in the room temperature structure. The other dominant packing interactions are weak hydrogen bonds between the CH groups of neighbouring aromatic rings and the sulphur atoms of the NCS groups; these interactions have been linked to the propagation of the spin transition through the crystal network and are found to be shorter in the trapped high-spin state. The observation that both the major intermolecular bonding interactions are found to be shorter in the trapped high-spin state than in the corresponding room temperature high-spin structure, implies an increased degree of cooperativity in the thermally trapped state.
3 Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridine and its derivatives
We have studied the parent compound of this series, Fe(bpp1)2](BF4)2 (3, where bpp1 = 2,6-di(pyrazol-1-yl)pyridine, Fig. 3), in great depth; it undergoes a complete, abrupt thermal spin transition with a small hysteresis loop, centred at 260 K. A detailed variable temperature crystallographic study of 3 showed that the compound remains in the monoclinic space group, P21, between 300 and 30 K, and the change in unit cell volume due to the spin transition alone was estimated to be 2.4% [20]. Interestingly, changing the counter anion in the compound from BF4– to PF6– suppresses the spin transition completely, since [Fe(bpp1)2](PF6)2 (4) is found to remain in the high-spin state over the whole temperature range. It was proposed that this was due to the C2 symmetry of the cation in the PF6– analogue causing loss of degeneracy of the iron d-orbitals [20]. Compound 3 exhibits a LIESST transition on irradiation with red light at low temperature to give a metastable high-spin state [21]. The crystal structure of this metastable state determined at 30 K, showed that there was an increase in unit cell volume of 49.3 Å3 (2.3%), which is in good agreement with estimations made from the variable temperature crystallographic study performed by Holland et al. [20]. Monitoring the unit cell parameters on warming after irradiation, showed that relaxation back to the ground low-spin state took place between 80 and 85 K, which is in good agreement with the photomagnetic data [22]. The structure of the cation in the metastable high-spin state at 30 K and in the thermal high-spin state at room temperature, is essentially the same, within experimental error, unlike the results for 1 reported by Marchivie et al. [14] discussed above, in which significant differences were reported between the structure of the metastable high-spin state and that determined at room temperature.

The low-spin state of [Fe(bpp1)2](BF4)2 (3) from X-ray data recorded at 30 K.
Photomagnetic data demonstrate that a number of iron(II) complexes with ligands derived from the bpp1 ligand show LIESST behaviour on irradiation and these include not only 3, but also [Fe(bpp2)2](ClO4)2 and [Fe(bpp2)2](BF4)2 (4 and 5, where the bpp2 = (2,6-dipyrazol-1-ylpyrazine, Scheme 2) [22], and [Fe(bpp3)2](BF4)2 and [Fe(bpp3)2](ClO4)2 (6 and 7, where bpp3 = 2,6-dipyrazol-1-yl-4-hydroxymethylpyridine, Scheme 2) [23,24]. The critical LIESST temperatures vary from 100 K for 5 to 66 K for 6 and under constant irradiation, all the compounds show Light Induced Thermal Hysteresis (LITH) [25] of varying magnitude, which emphasises the strongly cooperative nature of the metastable high-spin state.
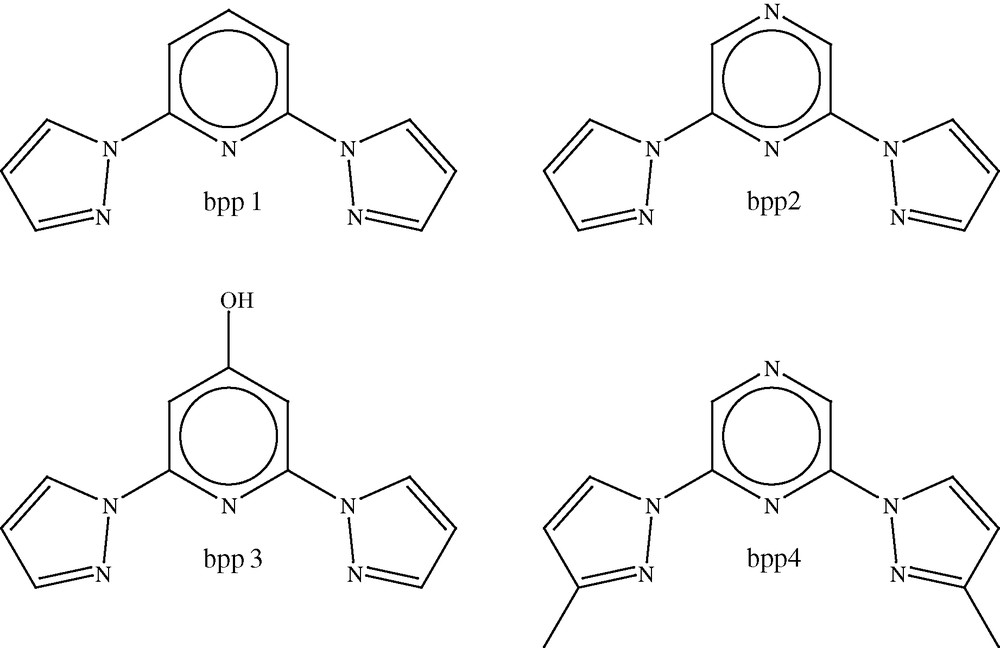
Tridentate ligands used in [Fe(L)2](X)2 SCO complexes.
As might be expected for materials in which a physical change is accompanied by a change in volume, SCO materials have been found to be extremely sensitive to changes in pressure. However the effect of increased, or reduced, pressure on the structure of this class of materials remains relatively poorly studied due to the intrinsic difficulties associated with the collection of data in these conditions and the complicated nature of many SCO compounds. Preliminary analysis of results of a single crystal X-ray diffraction experiment on 3 in a diamond anvil cell [26,27], shows that it is possible to induce the spin transition at room temperature by application of moderate pressure (~1.5 kbar). The effect of raised pressure on the thermal spin transition of 3 has also been monitored using SQUID magnetometry [28].
One of the primary areas of interest in the field of SCO research is the investigation of the role that cooperativity plays in determining the course of the spin transition. [Fe(bpp4)2](BF4)2 and [Fe(bpp4)2](ClO4)2 (8 and 9, where bpp4 = 2,6-di(3-methylpyrazol-1-yl)pyrazine), show unusual spin transition curves with a change in gradient at the point at which the transition is 50% complete, and although the curves are initially steep, at this temperature they become more gradual. In the case of 9, the transition occurs over a range of 210–90 K and the change in gradient is at 190 K. 8 has a change in gradient at 240 K with the spin transition taking place between 280 and 130 K. A detailed variable temperature crystallographic study revealed that the compounds crystallise in the tetragonal space group I over the whole temperature range under consideration. There is no evidence of ordering of the two spin states at the temperatures where the transitions are 50% complete.
9 was found to be merohedrally twinned about the twofold axis of the tetragonal unit cell. Monitoring the change in unit cell parameters with temperature showed that, although the unit cell volume and crystallographic a cell parameter follow the same trend with temperature as the magnetic data, the c cell parameter behaves in a completely different fashion. There is an increase in the c cell parameter on cooling over that temperature range, over which the spin transition curve shows a steep decrease in magnetisation. This is found to coincide with the temperatures at which the perchlorate anion, which is disordered at room temperature, becomes ordered. This ordering of the anion decreases the amount of intermolecular bonding in the system and the increase in the c cell parameter moves the iron centres slightly further apart; the result of these two effects is to reduce the ease with which the spin transition may be transmitted through the crystal network, i.e. decreases the cooperativity, resulting in a decrease in the rate of the SCO and hence a more gradual spin transition curve is observed. As far as we are aware, this is the only example of a SCO compound in which the behaviour of the anions are so closely linked to the form of the spin transition curve and in which this link can be so clearly demonstrated [29,30]. 8 and 9 also show LIESST behaviour on irradiation with red laser light at low temperature, however, relaxation to the low-spin state is too fast (even at 30 K), to permit full structural analysis.
4 Thermal and light induced polymorphism
All the complexes discussed above do not undergo a change in symmetry on cooling through the spin transition. This is quite unusual, since before 1986, SCO transitions with hystereses were generally associated with crystallographic phase changes [31]. However, we have recently reported a structural study involving two SCO materials, Fe[H2B(pz)2]L2 (where [H2B(pz)2]– = di(hydro)bis(1-pyrazolyl)borate and L = 2,2′-bipyridine (bipy, 10) or 1,10-phenanthroline (phen, 11), Scheme 1). Both 10 and 11 undergo SCO [32], but the transition is more gradual and takes place without hysteresis in the former. In both Fe[H2B(pz)2]bipy2, and Fe[H2B(pz)2]phen2, the SCO transition takes place at around 160 K on cooling, however, in 11, the transition is considerably more abrupt and takes place with a 4 K hysteresis. Photomagnetic data collected on both compounds indicate that they undergo LIESST [33], but TLIESST is significantly lower for Fe[H2B(pz)2]phen2, 11, than for Fe[H2B(pz)2]bipy2, 10, (44 K compared with 52 K). This is counter to previous experience, since in general, the more stable high-spin states (i.e. compounds with lower thermal transition temperatures), have higher LIESST relaxation temperatures.
X-ray diffraction data were collected by Real et al. [32] on 10 both above and below the SCO transition, however, in the case of 11 this was not possible as the crystals suffered structural damage on cooling. More recently, however, we have collected data on both compounds, above and below the transitions and have also succeeded in collecting data on both the LIESST states [34,35]. These experiments have brought to light the fact that the phenanthroline complex (11) undergoes a symmetry change, from C2/c to P on cooling through the SCO transition. This change in space group leads to a loss of the C2 molecular symmetry, as well as a loss of the C-centring, leading to a unit cell of approximately half the size of that seen above the transition. Spin transitions coupled with symmetry changes are not new, nonetheless the structure of the LIESST state generated from such a compound had not been determined until now. In this case, in the LIESST state the symmetry of the low-spin state is retained, while the iron centre is converted to high-spin. Thus, irradiating this complex at 30 K makes it possible to access a new high-spin state, with a different crystal structure from that of the thermal high-spin state (Fig. 4). We believe this is because the laser provides sufficient energy to excite the iron centre, but insufficient to enable the symmetry change. This also explains the TLIESST anomaly, since this new state can be assumed to be less stable than the thermal high-spin state, and therefore, relaxes sooner than the theoretical C2/c high-spin state.

Overlay of the two high-spin states of Fe[H2B(pz)2]phen2 (11), with the LIESST state shown as a broken line. The loss of the C2 symmetry can clearly be seen in the change in position of the [H2B(pz)2]– ligands.
5 SCO in bimetallic polymer networks
As demonstrated by the work discussed above, cooperativity in the solid state is intrinsically related to the communication between iron centres through weak intermolecular interactions. Attempts to increase the efficiency of this communication have led to the development of polymeric SCO compounds. A wide variety of these materials have been discussed in the literature since 1986 [36] and we have studied recently the structural characteristics primarily of bimetallic polymer networks, which utilise [M(CN)2]– bridging ligands (M = Ag, 12 and Au, 13), together with pyrimidine (pmd) ligands to link the iron centres together [37–40].
{Fe(pmd)2Fe(H2O)2[M(CN)2]4}·H2O is one of the most interesting of these materials and consists of two crystallographically and chemically distinct iron centres [40]. Both iron centres are coordinated equatorially by four linear [M(CN)2]– ligands, with the apical sites occupied by pyridine (Fe1) and water (Fe2). These iron centres alternate to form three interpenetrating networks consisting of –C≡N–Fe1–N≡C–M–C≡N–Fe2–N≡C–M– chains, which are in turn connected through weaker M–M interactions and hydrogen bonds (Fig. 5). Both the silver and gold analogues (12 and 13) undergo abrupt partial SCO transitions, which take place specifically at the Fe1 site. Both transitions take place with an 8 K hysteresis, but the transition in the gold polymer, 13, occurs at 165 K, while the silver polymer, 12, undergoes SCO at 218 K. On heating, both compounds progressively loose water, pyrimidine and cyanide. The dehydrated species can be trapped in both cases either by heating, or under vacuum to give two new compounds with differing properties: the dehydrated silver compound (formerly 12), undergoes SCO at 124 K with a hysteresis of 17 K, while the corresponding gold compound (formerly 13), does not. Thermogravimetric analysis carried out on these compounds indicates that they loose two molecules of water on heating to 400 K, but that the dehydration takes place in a destructive manner leading to the formation of crystalline powders. Thus, powder diffraction was necessary to characterise these materials structurally. In situ diffraction experiments on the gold sample, 13, upon heating, evacuating and rehydrating, indicate that it undergoes a completely reversible transition, characterised by a contraction of the a-axis of approximately 2.5 Å. Examination of the systematic absences indicate that the symmetry is retained (monoclinic, P21/c), and structure refinement confirms the loss of not only the solvent water, but also the ligand water, which enables the free nitrogen from the pmd to coordinate to the newly vacated water site on Fe2. Similar studies carried out on 12 suggest that the dehydration is also reversible, but the crystallinity of the samples is not as high and this, together with indications that there is a loss of symmetry, makes structural characterisation considerably more complex. Nonetheless it is clear, that the silver analogue undergoes a similar transformation, but the subtle structural differences mean that the SCO transition is retained with an enhanced hysteresis, suggesting the cooperativity is increased on dehydration.

Building the triply interpenetrated networks in {Fe(pmd)2Fe(H2O)2[M(CN)2]4}·H2O (12 and 13). The FeN6 and FeN4O2 octahedra are connected using [M(CN)2]– ligands to form sheets that are connected together at X and Y to form three interpenetrating networks bridged by M–M interactions and hydrogen bonding chains. When the water is lost, the hydrogen bonding network is destroyed and replaced by a second pmd → Fe bond (the M–M interactions are retained).
6 Conclusions
This paper describes the results of detailed structural studies on a cross section of the materials characterised in Durham over the past few years. We have shown that communication between iron centres, through intermolecular interactions or covalent bonds, is critical in determining the course of the spin transition. Moreover the examples discussed above illustrate the dramatic effect that subtle changes, such as in the identity of a counter ion (3), or the substitution of a silver atom with gold (compounds 12 and 13), can have on the physical properties of materials. Without considering fully the effect these minor changes have on the whole structure of the material, it is impossible to understand the relationship between the chemical compound and its physical properties. Thus, correlating the changes in physical behaviour with subtle changes in structure with a view to enhancing our understanding of these effects, is the best way to design new materials with specific and exploitable properties.
Our structural studies on SCO compounds have increased the wealth of knowledge within this fascinating area. However, it is clear that many important questions remain unanswered, which we believe will only be unravelled with the aid of very careful and detailed structural studies such as those described herein.
Acknowledgements
We would like to thank all our collaborators over the years, in particular Philippe Guionneau and Jean-François Létard (Bordeaux), Malcolm Halcrow (Leeds), Andrew Harrison and Simon Parsons (Edinburgh), and José Real and Carmen Muñoz (Valencia), together with their students past and present, without whom all of the above would not have been possible. In addition, we would also like to thank the EPSRC for postgraduate fellowships (ALT and VAM) and a Senior Research Fellowship (JAKH).


