Self-assembly of inorganic precursors in a quest for supramolecular chemistry is one of the highly recognized areas of chemical research [1–4]. The significant contemporary interest in polyoxovanadium-based solid materials reflects their diverse applications in the areas such as catalysis [1,2], materials science [2g], magnetism [1,5] and non-linear optics [1,2]. In studies of these materials, an astonishing variety of novel phases arises from the combination of vanadium progenitors and organic molecules [6]. Such structural variety arises from the versatility of vanadium in terms of its variable oxidation state (III, IV and V) and coordination geometry (tetrahedral, square pyramidal and octahedral), combined with the structure directing and stabilizing effect of the organic molecules. The combination of vanadium POMs with organic molecules adds complexity to the structures and promotes the adjustment of the physicochemical properties of these materials. Moreover, investigations on POM-organic/inorganic systems well characterized at molecular level, might provide structural and spectroscopic models [7] for heterogeneous metal oxide-supported catalysts. In comparison to the extensive work on polyoxometal-phosphates [8] only a very few polyoxometal-sulfites have been reported [9]. Being successful in synthesizing and characterizing polyoxometal-sulfites, we decided to prepare organic/POM-sulfite species. Herein, we report the synthesis, structural and physicochemical characterization, of the first organic/inorganic polyoxometal-sulfite compound (NH4)3(4,4´-Hbpy)[(VIVO)6(μ4-O)2(μ3-OH)2(μ3-SO3)4(μ-SO3)]·15/2 H2O 1.
Solid (NH4)2SO3 (6.00 g, 61.2 mmol) was added in one portion to a stirred solution of NH4VVO3 (0.60 g, 5.1 mmol, pH 0) in concentrated (37%) HCl/H2O (1:4 v/v, 20 ml). Upon addition of (NH4)2SO3 the light-yellow color of the solution changed to blue–green, and its pH value was ca. 4. After stirring the solution for ~15 min, it was filtered and an ethanolic solution (6 ml) containing 4,4′-bpy (0.40 g, 2.5 mmol) was layered on it. A layer of diethyl ether was placed between these two layers. Green hexagonal crystals of 1, suitable for X-ray crystal structure analysis1, were obtained after 3 days. Yield 0.48 g (31%) based on vanadium. Compound 12 is indefinitely stable in air.
The structure of compound 1 consists of a one-dimensional extended network constructed by vanadium building-blocks, [H2V6IVS4IVO22]2–/SO32–, protonated bipyridine (4,4′-Hbpy+) as well as ammonium cations and interstitial water molecules. Neighboring [H2V6IVS4IVO22]2– shells are linked together by the pyramidal μ2-(O,O) bridging sulfite groups to form infinite chains running along [001]. Two such chains are linked into a ‘double-rung ladder’ assembly via two different hydrogen bonds provided by singly protonated bipyridine cations (Fig. 1). The principal interatomic distances and selected band angles for 1 are reported in Table 1. The 4,4′-Hbpy+ is nearly planar with the two pyridyl rings twisted by 11.6(4)°. One strong hydrogen-bond is formed between H(9) [of the triply-bridging O(9) hydroxo ligand] and the N(1) atom of 4,4′-Hbpy+ [O(9)···N(1), 2.661(4) Å, 179.0°]. Another hydrogen bond is formed between H(2) of 4,4′-Hbpy+ and O(4) {oxo-ligand of the vanadium cluster; [N(2)···O(4), 2.731(6) Å, 174.8°]}. Packing between ladder ribbons is achieved by an extensive hydrogen-bonding system in which all ammonium cations and water molecules are involved. The structure of the vanadosulfite building block (Fig. 2), [H2V6IVS4IVO22]2–, is essentially identical to that previously reported [9b]. The cluster consists of a distorted cubane unit, [H2V4O4], which is connected to two outer distorted square-pyramidal vanadium(IV) atoms through two μ4-O2– bridges and four μ3-SO32– bridges as well. Bond valence sum (BVS) [10] calculations (Table 2) for the crystallographically independent vanadium atoms gave values in the range of 3.94–4.04 suggesting that all vanadium atoms are in the oxidation state of IV. BVS calculation [10] for the triply bridging oxygen atoms [O(9) and O(10)], gave values of 1.06 and 1.05, respectively, indicating single protonation according to elemental analysis and charge balance, while the sulfur atoms gave values very close to 4.

Ball-and-stick representation of compound 1.
Principal interatomic distances (Å) and selected bond angles (°) for the compound 1
| Bond lengths (Å) | |||||
| V(1)–O(1) | 1.607(3) | V(3)–O(3) | 1.596(3) | V(5)–O(5) | 1.594(3) |
| V(1)–O(7) | 1.989(3) | V(3)–O(9) | 1.984(3) | V(5)–O(19) | 1.971(3) |
| V(1)–O(8) | 1.990(3) | V(3)–O(10) | 1.999(3) | V(5)–O(13) | 1.974(3) |
| V(1)–O(14) | 2.034(3) | V(3)–O(18) | 2.031(3) | V(5)–O(23) | 1.976(3) |
| V(1)–O(11) | 2.036(3) | V(3)–O(12) | 2.046(3) | V(5)–O(7) | 2.002(3) |
| V(1)–O(9) | 2.260(3) | V(3)–O(7) | 2.255(3) | V(6)–O(6) | 1.591(4) |
| V(2)–O(2) | 1.599(3) | V(4)–O(4) | 1.607(3) | V(6)–O(24) | 1.972(3) |
| V(2)–O(8) | 1.996(3) | V(4)–O(10) | 1.988(3) | V(6)–O(22) | 1.973(3) |
| V(2)–O(7) | 1.998(3) | V(4)–O(9) | 1.993(3) | V(6)–O(16) | 1.991(3) |
| V(2)–O(17) | 2.025(3) | V(4)–O(21) | 2.011(3) | V(6)–O(8) | 2.005(3) |
| V(2)–O(20) | 2.030(3) | V(4)–O(15) | 2.022(3) | V(1)···V(2) | 2.8894(11) |
| V(2)–O(10) | 2.302(3) | V(4)–O(8) | 2.240(3) | V(3)···V(4) | 3.1304(11) |
| V(3)···V(5) | 3.6261(12) | ||||
| V(4)···V(6) | 3.6001(13) | ||||
| Bond angles (°) | |||||
| O(1)–V(1)–O(7) | 103.33(13) | O(20)–V(2)–O(10) | 76.84(11) | O(5)–V(5)–O(13) | 106.45(16) |
| O(1)–V(1)–O(8) | 103.91(14) | ||||
| O(7)–V(1)–O(8) | 85.58(11) | O(2)–V(2)–V(1) | 99.84(12) | O(19)–V(5)–O(13) | 142.93(14) |
| O(1)–V(1)–O(14) | 100.00(14) | O(8)–V(2)–V(1) | 43.47(8) | O(5)–V(5)–O(23) | 101.58(17) |
| O(7)–V(1)–O(14) | 156.55(12) | O(7)–V(2)–V(1) | 43.43(8) | O(19)–V(5)–O(23) | 84.69(13) |
| O(8)–V(1)–O(14) | 91.09(11) | O(17)–V(2)–V(1) | 132.74(9) | O(13)–V(5)–O(23) | 80.56(13) |
| O(1)–V(1)–O(11) | 99.24(14) | O(20)–V(2)–V(1) | 133.33(8) | O(5)–V(5)–O(7) | 102.08(14) |
| O(7)–V(1)–O(11) | 91.28(11) | O(10)–V(2)–V(1) | 83.92(7) | O(19)–V(5)–O(7) | 90.01(12) |
| O(8)–V(1)–O(11) | 156.74(12) | O(3)–V(3)–O(9) | 102.07(14) | O(13)–V(5)–O(7) | 90.05(12) |
| O(14)–V(1)–(11) | 82.69(11) | O(3)–V(3)–O(10) | 104.07(15) | O(23)–V(5)–O(7) | 156.17(15) |
| O(1)–V(1)–O(9) | 176.56(13) | O(9)–V(3)–O(10) | 74.96(11) | O(6)–V(6)–O(24) | 103.55(19) |
| O(7)–V(1)–O(9) | 78.92(10) | O(3)–V(3)–O(18) | 98.10(15) | O(6)–V(6)–O(22) | 107.69(17) |
| O(8)–V(1)–O(9) | 78.76(11) | O(9)–V(3)–O(18) | 157.38(12) | O(24)–V(6)–O(22) | 86.21(14) |
| O(14)–V(1)–O(9) | 77.67(11) | O(10)–V(3)–O(18) | 90.34(12) | O(6)–V(6)–O(16) | 106.52(18) |
| O(11)–V(1)–O(9) | 78.01(11) | O(3)–V(3)–O(12) | 95.75(16) | O(24)–V(6)–O(16) | 78.60(14) |
| O(1)–V(1)–V(2) | 99.08(11) | O(9)–V(3)–O(12) | 90.77(12) | O(22)–V(6)–O(16) | 144.95(15) |
| O(7)–V(1)–V(2) | 43.69(8) | O(10)–V(3)–O(12) | 157.52(12) | O(6)–V(6)–O(8) | 102.70(15) |
| O(8)–V(1)–V(2) | 43.62(8) | O(18)–V(3)–O(12) | 97.35(13) | O(24)–V(6)–O(8) | 153.38(17) |
| O(14)–V(1)–V(2) | 134.00(9) | O(3)–V(3)–O(7) | 176.30(15) | O(22)–V(6)–O(8) | 90.08(12) |
| O(11)–V(1)–V(2) | 134.27(8) | O(9)–V(3)–O(7) | 79.12(10) | O(16)–V(6)–O(8) | 89.75(12) |
| O(9)–V(1)–V(2) | 84.35(7) | O(10)–V(3)–O(7) | 79.60(11) | V(1)–O(7)–V(2) | 92.89(11) |
| O(2)–V(2)–O(8) | 104.06(14) | O(18)–V(3)–O(7) | 81.40(11) | V(1)–O(7)–V(5) | 120.77(13) |
| O(2)–V(2)–O(7) | 104.27(14) | O(12)–V(3)–O(7) | 80.71(11) | V(2)–O(7)–V(5) | 120.48(13) |
| O(8)–V(2)–O(7) | 85.17(11) | O(4)–V(4)–O(10) | 103.36(15) | V(1)–O(7)–V(3) | 100.28(11) |
| O(2)–V(2)–O(17) | 100.47(14) | O(4)–V(4)–O(9) | 104.05(15) | V(2)–O(7)–V(3) | 101.16(11) |
| O(8)–V(2)–O(17) | 155.43(12) | O(10)–V(4)–O(9) | 75.02(11) | V(5)–O(7)–V(3) | 116.67(12) |
| O(7)–V(2)–O(17) | 90.16(11) | O(4)–V(4)–O(21) | 97.62(15) | V(1)–O(8)–V(2) | 92.91(11) |
| O(2)–V(2)–O(20) | 100.33(14) | O(10)–V(4)–O(21) | 92.18(12) | V(1)–O(8)–V(6) | 120.99(14) |
| O(8)–V(2)–O(20) | 90.76(11) | O(9)–V(4)–O(21) | 156.82(12) | V(2)–O(8)–V(6) | 120.96(14) |
| O(7)–V(2)–O(20) | 155.32(12) | O(4)–V(4)–O(15) | 94.21(15) | V(1)–O(8)–V(4) | 100.78(12) |
| O(17)–V(2)–O(20) | 83.48(11) | O(10)–V(4)–O(15) | 158.62(12) | V(2)–O(8)–V(4) | 100.79(12) |
| O(2)–V(2)–O(10) | 176.23(13) | O(9)–V(4)–O(15) | 89.07(12) | V(6)–O(8)–V(4) | 115.90(12) |
| O(8)–V(2)–O(10) | 78.60(11) | O(21)–V(4)–O(15) | 97.54(14) | V(3)–O(9)–V(4) | 103.85(12) |
| O(7)–V(2)–O(10) | 78.49(10) | O(4)–V(4)–O(8) | 175.57(14) | V(3)–O(9)–V(1) | 100.28(11) |
| O(17)–V(2)–O(10) | 76.83(11) | O(10)–V(4)–O(8) | 80.29(11) | V(4)–O(9)–V(1) | 100.02(12) |
| O(9)–V(4)–O(8) | 79.21(11) | V(4)–O(10)–V(3) | 103.47(12) | ||
| O(21)–V(4)–O(8) | 79.62(12) | V(4)–O(10)–V(2) | 98.95(12) | ||
| O(15)–V(4)–O(8) | 82.79(12) | V(3)–O(10)–V(2) | 99.57(11) | ||
| O(5)–V(5)–O(19) | 109.75(16) |
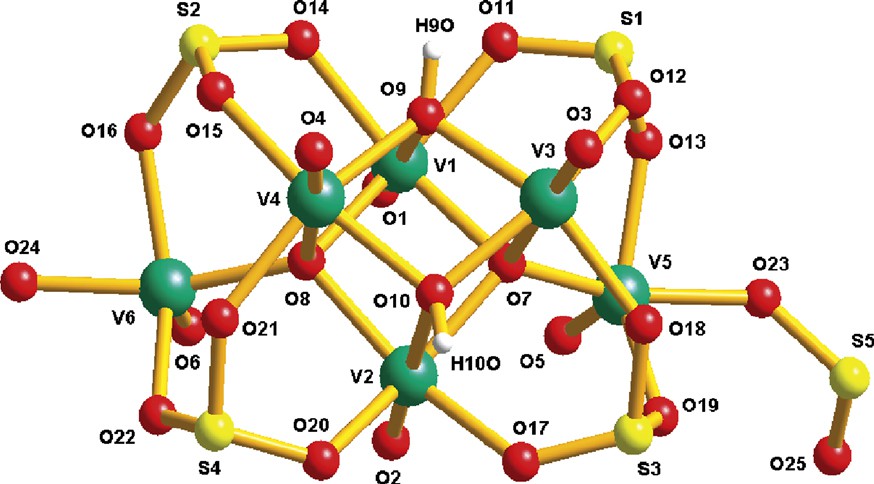
Ball and stick representation of the vanadium building block, [H2V6IVS4IVO22]2– in 1. Average bond lengths (Å): V=O 1.599(3), V–O (μ3-O) 2.096(3), V–O (μ4-O) 2.107(3), S–O (μ3-SO32–) 1.532(3), S=O 1.446(4).
Bond valance sum calculations for the vanadium, sulfur and oxygen atoms for the compound 1
| Atom | BVS | Atom | BVS | Atom | BVS |
| V(1) | 3.99 | O(7) | 1.89 | O(19) | 1.54 |
| V(2) | 4.00 | O(8) | 1.59 | O(20) | 1.55 |
| V(3) | 4.03 | O(9) | 1.06 | O(21) | 1.54 |
| V(4) | 4.04 | O(10) | 1.07 | O(22) | 1.55 |
| V(5) | 3.95 | O(11) | 1.63 | O(23) | 1.62 |
| V(6) | 3.94 | O(12) | 1.75 | O(24) | 1.74 |
| O(1) | 1.65 | O(13) | 1.55 | O(25) | 1.48 |
| O(2) | 1.69 | O(14) | 1.54 | S(1) | 3.97 |
| O(3) | 1.71 | O(15) | 1.54 | S(2) | 3.95 |
| O(4) | 1.65 | O(16) | 1.55 | S(3) | 3.97 |
| O(5) | 1.72 | O(17) | 1.54 | S(4) | 3.87 |
| O(6) | 1.74 | O(18) | 1.55 | S(5) | 3.96 |
The infrared spectrum of 1 exhibits bands at 3504s (br) [v(O–H) from H2O], 3127s (br) [v(N–H) from NH4+], 1637m (br) [δ(H2O)], 1607m, 1560w, 1211w [ν(4,4´-Hbpy+)], 1401s [δ(NH4+)], 1028sh, 922s, 874s, 834s, 814m and 568s cm–1 [v(SO32–)] [11], 979s and 971sh cm–1 [v(V=O)], respectively. The electronic spectrum of 1 in aqueous solution consists of a band in the visible and a peak in the ultraviolet region, namely, λ/nm(ɛ/M–1 cm–1)]: 889(sh) (1261), 251 (18,440). The UV–Vis solid state reflectance of compound 1, revealed a band at 251 nm, thus indicating that 1 loses its integrity in aqueous solution.
The experimental magnetic data for 1 are plotted as χMT vs. T in Fig. 3A. The χMT value decreases from 1.74 emu mol–1 K at 300 K, which is significantly smaller with the expected value of 2.25 emu mol–1 K for six non-interacting VIV centers (S = 1/2), to 1.53 emu mol–1 K at 78 K and then increases abruptly to the value of 1.70 emu mol–1 K at 5.8 K and then drastically decreases to the value of 1.48 at 1.8 K. The behavior of χMT suggests the existence of very strong antiferromagnetic interactions along with ferromagnetic exchange interactions within the molecule. The Hamiltonian formalism used to fit the experimental data for this VIV6 system (Scheme 1) is given by Eq. 1.(1)

A) Temperature dependence of the susceptibility data, in the form of χMT vs. T for 1. The solid line represents the fitting results according to equation 1; B) magnetization data for 1 at 2.5 and 4.5 K.

In conclusion, the successful isolation of a novel one-dimensional species (NH4)3(4,4′-Hbpy)[(VIVO)6(μ4-O)2(μ3-OH)2(μ3-SO3)4(μ-SO3)]·15/2 H2O 1, provides a novel example of assembling vanadosulfite with sulfite anion through the structure-control effect of 4,4′-Hbpy+ cation. In addition, compound 1 is the first organic/inorganic polyoxometal-sulfite species in which the 4,4′-Hbpy+ ligand is only involved in hydrogen-bonding. Variable temperature magnetic susceptibility measurements revealed an overall antiferromagnetic behavior for 1, in marked contrast to the ferromagnetic behavior for the previously described hexanuclear species [H2V6IVS4IVO22]2– [9b].
Acknowledgments
This research was funded by the program ‘Heraklitos’ of the Operational Program for Education and Initial Vocational Training of the Hellenic Ministry of Education under the 3rd Community Support Framework and the European Social Fund.
1 Elemental analysis calcd. (%) for C10H38N5O32.5S5V6 (1214.39): C: 9.88, H: 3.12, N: 5.76, S: 13.17, V: 25.17; found: C: 9.81, H: 3.04, N: 5.63, S: 12.98, V: 25.01.
2 X-Ray crystal structure analysis for 1: (NH4)3(4,4′-bipyH)[(VIVO)6(μ4-O)2(μ3-OH)2(μ3-SO3)4(μ-SO3)]·15/2 H2O: C10H38N5O32.5S5V6, Mr = 1214.39, triclinic, space group P


