1 Introduction
Alkali metal containing low dimensional chalcogenide compounds may exhibit very fascinating chemical reactivity in solution. Hence, LiMo3Se3 inorganic material containing infinite 1/∞[Mo3Se3]– chains is soluble in highly polar organic solvents such as dimethylsulfoxyde (DMSO) and N-methylformamide (NMF) to form colloidal solutions [1]. Many studies including optical microscope under polarized light measurements have confirmed the lyotropic nematic phase behavior of LiMo3Se3 in NMF solution [2,3]. More recently, the two isostructural materials KNiPS4 [4] and KPdPS4 [5] have been synthesized. Both compounds enclose infinite 1/∞[MPS4]– chains built upon [MS4] squares and [PS4] tetrahedra sharing edges, and are soluble in polar and donor organic solvents. Optical microscopy under polarized light and transmission electron microscopy studies have been shown that KPdPS4/DMF solutions exhibit a fluid complex behavior due to the maintaining of spaghetti chains in solution [6,7]. At the opposite, the irreversible lost of the transient birefringence of few hours aged KNiPS4/DMF solutions have been highlighted [6]. 31P NMR spectroscopy [6] and mass spectrometry [7] have revealed that the infinite 1/∞[NiPS4]– chains are subjected to a solvent-induced fragmentation/cyclisation process in solution that leads to discrete crown-shaped [Ni3P3S12]3– entities (Figs. 1 and 2). Both the 0D and 1D species containing nickel thiophosphates have been characterized in solid state and in solution. These studies evidenced that 31P NMR spectroscopy is very well adapted in detecting the presence of [Ni3P3S12]3– anions and infinite 1/∞[NiPS4]– chains: the later exhibits one characteristic resonance peak located at 119.6 ppm in solid state and at 122 ppm in DMF solution, whereas [Ni3P3S12]3– species give rise to a sharp or a broad resonance peak at about 114 ppm for solution and solid NMR measurements, respectively (in the solid state, the broad peak may sometimes be decomposed in three, four or more contributions versus the three dimensional packing of the [Ni3P3S12]3– anions and the symmetry operations at work) [6–8].

(a) Infinite 1/∞[NiPS4]– chains in KNiPS4. (b) View of discrete [Ni3P3S12]3– complex along its pseudo threefold axis.

Scheme showing the versatile reactivity of [Ni3P3S12]3– complex in DMF solution. See text for explanations.
It is now clear that several parameters may control the exfoliation of KNiPS4 in solution. Hence, the kinetic of the cyclisation-fragmentation of the infinite 1/∞[NiPS4]– chains into [Ni3P3S12]3– discrete entities strongly depends on the KNiPS4 precursor concentration, the temperature, the possible use of a macrocyclic molecule, and of course, the nature of the solvent (polar organic solvent with high dielectric constant will favor dissolution). The same applies to the reactivity of [Ni3P3S12]3– anions in solution. Hence, under the influence of the organic countercations used to exchange K+ for instance, the stabilization of the discrete anionic [Ni3P3S12]3– species in solid state can achieved or 1/∞[NiPS4]– chains can be reconstructed. In Fig. 2 is illustrated the versatile behavior of [Ni3P3S12]3– in solution.
1 – The use of organic cryptands allows a better solubilization of KNiPS4 by complexing the alkali cations and promoting the metathesis reactions. In this way, the reactivity of macrocyclic molecule 4,7,13,16,21,24-hexaoxa-1,10-diazobicyclo[8.8.8]hexacosane (222-cryptand) (specially suitable for the complexation of K+ ions) towards KNiPS4/DMF solution leads to the formation of the [K-(222-cryptand)]3[Ni3P3S12] compound characterized in the solid state by X-ray diffraction [8]. In this material, [Ni3P3S12]3– containing infinite 2/∞[NiPS4]– layers are well-separated by the cryptands-encapsulated K+ ions. On this geometrical point of view, the [K-(222-cryptand)]3[Ni3P3S12] structural arrangement reminds the one of KNiPS4 where 1/∞[NiPS4]– chains are condensed in infinite slabs.
2 – [Ni3P3S12]3– anions can be also stabilized in the solid state by replacing K+ ions by isotropic organic counter-cations such as tetraalkylammonium NR4+ (R = Me, Et, Bu) or tetraphenylphosphonium (PPh4+) [6,7]. As for [K-(222-cryptand)]3[Ni3P3S12], this may be related to the classical steric effect observed in inorganic materials [9–11]: the dimensionality of the anionic framework decreases from (1D) 1/∞[NiPS4]– chains to (0D) [Ni3P3S12]3– complexes when the size of the monovalent countercations increases.
3 – When the exchange of K+ by monovalent anisotropic cations such as hexyltrimethylammonium [CH3(CH2)5N(CH3)3]+ and dodecyltrimethylammonium [CH3(CH2)11N(CH3)3]+ ions is performed with the help of 222-cryptand, the [Ni3P3S12]3– entities are stabilized in the solid state, whereas without macrocyclic molecule, the anionic complexes shift to the restacking of the 1/∞[NiPS4]– chains [8]. This surprising versatile behavior of [Ni3P3S12]3– is still unexplained but the polymerization of crowns into infinite chains was already observed with temperature in liquid-crystalline films containing [Ni3P3S12]3– entities and charged double-tail dialkylammonium surfactants (the reverse process occurs by dissolution) [12].
4 – The restacking of the chains is also observed when the concentration of trinuclear complex increases. The drying of a [Ni3P3S12]3–/DMF solution gives rise to an amorphous material [13]. 31P solid state NMR spectrum of this compound have shown a lone broad-line located at δ = 121 ppm, characteristic of the presence of 1/∞[NiPS4]– polymer. This broadening, more emphasized in the restacked materials than in the KNiPS4 pristine material (Fig. 3), may be due to possible factors such as the amorphous state of the material, residual 31P–1H dipolar interactions with trapped solvent molecules or the occurrence of [NinPnS4n]n– linear fragments with a wide length distribution. So far, the mechanism leading to the reconstruction of the polymeric chains from the discrete trinuclear complexes remains mysterious. However, it appears that the concentration in minerals plays a major role in this reversible process.
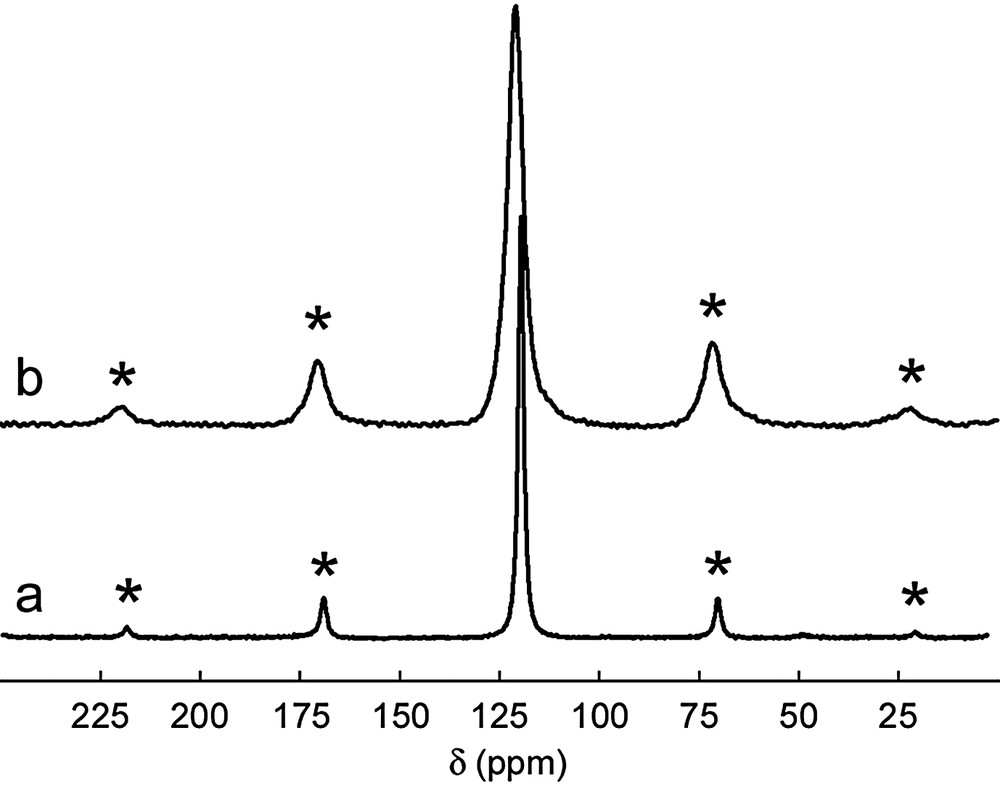
31P MAS NMR spectrums recorded at 300 K of crystallized KNiPS4 (a) and restacked material obtained from the drying of [Ni3P3S12]3–/DMF solution (b) (asterisks denote spinning side bands). The KNiPS4 spectrum presents one peak located around 119.6 ppm for (a) and 121.0 ppm for (b), corresponding to the phosphorus atoms inside the chain.
5 – In contrast to the ion-exchange process with monovalent organic cations, the reactivity of [Ni3P3S12]3–/DMF solution towards large sized bi-charged organic cations such as trimethylene bis(triphenylphosphonium) ((C6H5)3–P+–(CH2)3–P+–(C6H5)3) or 1,2-vinylene bis(triphenylphosphonium) ((C6H5)3–P+–CH=CH–P+–(C6H5)3) yields the reconstruction of 1/∞[NiPS4]– chains in the solid state. This has been proven by 31P-NMR, Raman and IR spectroscopies [14]. At first sight, this behavior is in disagreement with the expectation that the higher the size of the countercations, the lower the dimensionality of the anionic framework. However, in the present case, it appears that the steric effect of the organic cations may be counterbalanced by a charge effect, i.e. the higher charge of the organic ions goes along with a lesser concentration in countercation. Nevertheless, the positive charge of the aforementioned organic bi-cations is localized around the two, well-separated phosphorus atoms, and the organic cations may be then described to some extents as the condensation of two mono-charged cations. Hence, as the influence of the charge density of the dication on the reactivity remains unknown, it remains questionable whether the restacking of 1/∞[NiPS4]– chains is due to a charge effect or a steric effect of the cations. To unravel this question, the ion-exchange of K+ with localized bi-charged cations such as [Fe(o-phen)3]2+ has been performed. We report here the synthesis of [Fe(o-phen)3]1.5[Ni3P3S12] (1), a new heterobimetallic compound.
2 Experimental section
2.1 Chemicals
All manipulations were carried out under the oxygen-free dry nitrogen atmosphere using standard Schlenk techniques. All commercial solvents were used as received. 1,10-Phenanthroline·H2O (puriss 99%), FeSO4·7H2O (puriss 98%), NaBF4 (puriss 99%) and 18-crown-6 ether (puriss 98%) were purchased from Aldrich. KNiPS4 was prepared as previously described [15].
2.2 NMR spectroscopy
Solid state NMR experiments were performed at room temperature on a Bruker Avance 500 MHz operating at 202.4 MHz for 31P, using a 4-mm double-bearing Bruker probehead. 31P MAS spectra of KNiPS4 were acquired at 10 kHz and a repetition time of 10 s. 31P MAS spectrum of [Fe(o-phen)3]1.5[Ni3P3S12] (1) was acquired at 15 kHz with a repetition time of 20 s. Except for crystallized KNiPS4, 1H decoupling was systematically used during acquisition. Spectra were referenced to H3PO4.
2.3 Synthesis of (BF4)2[Fe(o-phen)3]
FeSO4·7H2O (278 mg, 1 mmol) and FeSO4 enriched in 57Fe (31 mg, 0.2 mmol) were dissolved in 20 ml of water. 1,10-Phenanthroline·H2O (793 mg – 4 mmol) was added and the resulting dark-red solution was stirred for few minutes. NaBF4 (550 mg – 5 mmol) was added and the solution was placed in a ice-bath leading to the formation of a red solid. The powder was isolated by filtration and dried under vacuum (yield: 80%).
2.4 Synthesis of [Fe(o-phen)3]1.5[Ni3P3S12] (1)
KNiPS4 (216 mg – 0.84 mmol) and 18-crown-6 ether (1,4,7,10,13,16-hexaoxacyclooctadecane) (0.204 mg – 0.85 mmol) was dissolved in 15 ml of DMF and the red-dark solution was stirring at 50 °C for 15 h. (BF4)2[Fe(o-phen)3] is then added to the solution leading to the formation of a air-stable red solid. The solution was filtered and the powder was washed with ethanol and acetone (yield: 65%). An EDXS (Energy dispersive X-ray spectroscopy) analysis of the heaviest elements by means of a Jeol microscope (PGT-IMIX-PTS equipped Jeol-JSM5800LV) yielded the elemental ratio Ni2.8/P3.0/S11.5/Fe1.5 in good agreement with the [Fe(o-phen)3]1.5[Ni3P3S12] title composition.
3 Results and discussion
Fe2+ cations react with three equivalent of 1,10-phenanthroline in aqueous solution to give a red octahedral low spin tris(phenanthroline) iron (II) complex. Addition of bi-charged [Fe(o-phen)3]2+ cation to KNiPS4/DMF solution leads to the formation of [Fe(o-phen)3]1.5[Ni3P3S12] (1) that precipitates as a red solid. The metathesis of K+ ions by [Fe(o-phen)3]2+ complexes is performed with the presence of 18-crown-6 ether to ensuring a better separation of charge in solution and also to increase anion's reactivity. The red powder of 1 is amorphous and no crystal suitable for X-ray diffraction characterization was obtained. The +2 oxidation state of Fe and its low spin configuration in 1 was confirmed by Mössbauer spectroscopy1. 31P NMR measurements were carried out to determine whether 1 contains 1/∞[NiPS4]– chains or [Ni3P3S12]3– entities. The 31P MAS NMR spectrum is shown on Fig. 4. It reveals the presence of a peak located at δ = 112 ppm. By comparison with the spectrum of (PPh4)3[Ni3P3S12], [6] this signal has been undoubtedly attributed to the phosphorus atoms of [Ni3P3S12]3– anions. No signal of the 1/∞[NiPS4]– chains at δ = 119.6 ppm appears. Contrary to the ion-exchange process with bi-charged organic cations such as trimethylene bis(triphenylphosphonium) or 1,2-vinylene bis(triphenylphosphonium) that led to 1/∞[NiPS4]– polymer, the substitution of K+ counterions by localized bi-charged cations such as [Fe(o-phen)3]2+ complexes allows the stabilization of [Ni3P3S12]3– crown-shaped anions both in solution and solid state. Consequently, the restacking of infinite 1/∞[NiPS4]– chains in (PPh3–C3H6–PPh3)0.5[NiPS4] and (PPh3–C2H2–PPh3)0.5[NiPS4] is not only due to a pure charge effect such as it was proposed initially but has to deal probably with the charge density distribution on the organic cations.
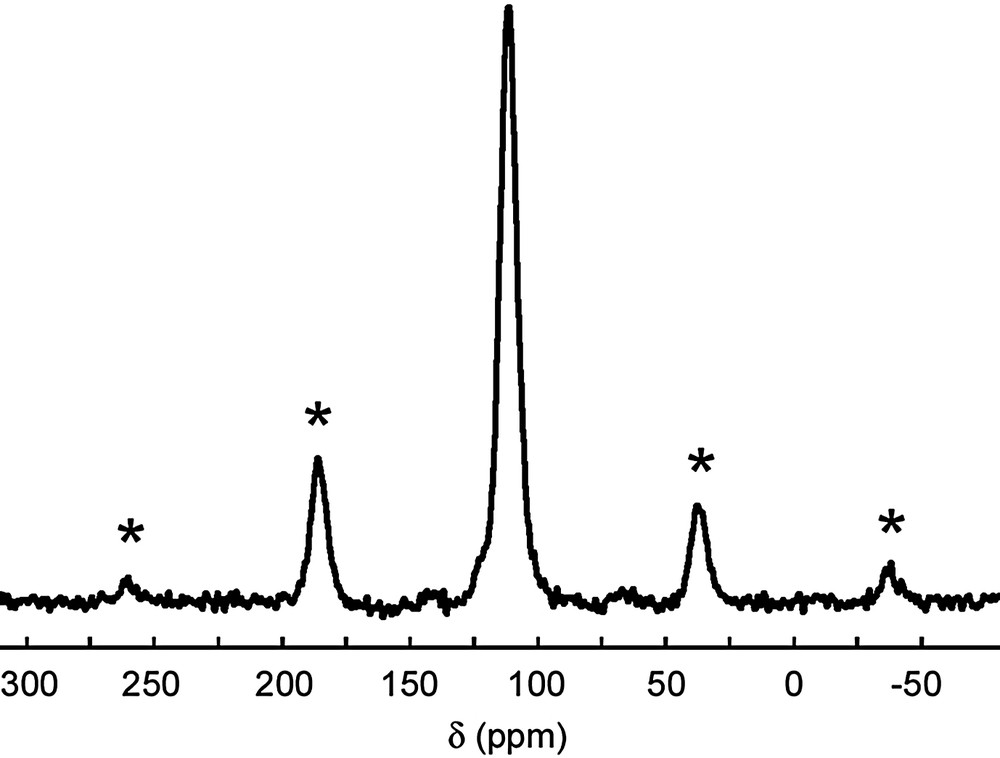
31P MAS NMR spectrum of [Fe(o-phen)3]1.5[Ni3P3S12] (1) recorded at 300 K. (asterisks denote spinning side bands). The signal of the phosphorus atoms of the [Ni3P3S12]3– complexes in 1 is located at δ = 112 ppm.
4 Conclusions
The versatile behavior of the [Ni3P3S12]3– complexes towards organic and inorganic cations in DMF solution has again been highlighted. The mechanism of dissolution/restacking of the (1D) 1/∞[NiPS4]– including the systematic formation of [Ni3P3S12]3– anions versus any other [NixPxS4x]x– (x > 3) is still unknown. The stabilization of discrete trinuclear entities or infinite 1/∞[NiPS4]– chains in solid state from the evaporation of [Ni3P3S12]3– anions containing solution cannot be only explained in term of a steric effect or a charge effect of the countercations.
1 Mössbauer spectra of [Fe(o-phen)3]1.5[Ni3P3S12] (1) have shown that the 57Fe signal is not perturbed by the trinuclear [Ni3P3S12]3– complexes, showing very weak interactions between anionic and cationic fragments in the network.


