1 Introduction
Sclerochiton vogelii (Nees) T. Anderson (Acanthaceae), is a herbaceous traditional medicinal plant used in Gabon by healers. The decoction from aerial parts is used for the treatment of hypertension and anemia. Until now, the chemistry of the plant has not been investigated. In this study, the isolation and structure elucidation of two new flavones: 7-O-{α-l-rhamnopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-β-d-glucopyranosyl} luteolin 1 and 7-O-β-d-apiofuranosyl (1 → 2)-β-d-xylopyranosyl luteolin 2 and one known flavone glycoside: 7-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl luteolin 3 are reported.
The structure of the compound 1 was elucidated using one- and two-dimensional NMR (COSY, HMQC and HMBC) and mass spectrometry, while the structure of 2 and 3 were determined by MS, 1H NMR, 13C NMR and COSY experiments. The spectral data for compound 3 were in good agreement with those previously reported in the literature [1]. This last compound is described for the first time in this plant.
2 Experimental
2.1 General experimental procedures
Optical rotations were measured on a Perkin Elmer, 341 OROT 589 nm Polarimeter.
UV spectra were recorded on a Spectrophotometer UV/Vis Beckman DU 520.
IR spectra were performed on a Nicolet 205 XB FTIR spectrometer.
Melting points were determined on a Buchi Melting point B 540 apparatus.
Mass spectra were obtained on a Finnigan LCQ quadrupole ion trap instrument fitted with an electrospray ionization source operated in the negative ion mode. Sample solutions (0.5 mg l–1 in CH3OH/H2O (50:50) were analyzed by infusion at a flow rate of 3 μl min–1. Hydrogen/deuterium exchange experiments were carried out by using CH3OD/D2O (50:50) as the solvent system instead of the non deuterated one. The electrospray needle was set at –4.5 kV. The heated transfer capillary was maintained at 200 °C. Other operating parameters were adjusted for each sample in order to optimize the signal and obtain the maximal structural information from the ion of interest. All spectra were acquired under automatic gain control (AGC) conditions using helium as buffering and collision gas for MS/MS experiments.
1H NMR, 13C NMR and 2D NMR spectra were determined on a Bruker Avance DRX-500 spectrometer operating at 500.13 MHz (1H NMR) and 125.75 MHz (13C NMR) in CD3OD (compounds 2 and 3) and DMSO-d6/TFAA (9:1) (compound 1). TMS was used as internal standard in 1H and 13C measurements. Standard Bruker pulse sequences were used for 2D experiments. Copies of the original spectra are obtainable from the correspondence author.
2.2 Plant material
A sample of the herbaceous S. vogelii was collected in Libreville, Gabon in April 1996. A voucher specimen (SV-4-1996, HNG 53) identified by H. Bourobou, botanist (National Herbarium of Gabon at the Institute of Traditional Pharmacopoeia and Medicine (IPHAMETRA) is kept in the Department of Pharmacognosy, Faculty of Pharmacy, Marseilles, France and in the herbarium of the IPHAMETRA Institute (Libreville, Gabon).
2.3 Extraction and isolation
The decoction was prepared by heating the dried plant (1 kg) at 100 °C in H2O during 15 min. After decantation and filtration, the H2O layer was freeze dried. The aqueous extract (5 g) was partitioned on polyamide with a gradient of acetone, ethanol, MeOH in H2O: H2O (1.5 l), MeOH 20% (1.5 l), MeOH 40% (1.5 l), MeOH 50% (1.5 l), MeOH 60% (1.5 l), MeOH 80% (1.5 l), MeOH (2 l), ethanol (1 l), acetone/H2O (80:20) (2 l).
According to the differences in composition monitored by TLC 12 fractions were obtained (F1–F12).
F6 was subjected to silica gel chromatography, eluting under isocratic conditions with EtOAc/iso-PrOH/H2O (65:25:5). Five fractions were obtained (A–E).
Fractions B and D were subjected individually to gel filtration on Sephadex LH-20 and elution with acetone.
Flavone 3 (14 mg), a pure yellow compound, was obtained from fraction B (130 mg).
Fraction D (50 mg) was chromatographed and yielded the pure flavone 2 (28 mg).
F9 (1 g) was applied to a silica gel column, eluting under isocratic conditions with EtOAc/iso-PrOH (65:25). Seven fractions were obtained (F–L).
Fraction G (300 mg) was partitioned on polyamide with MeOH–H2O (gradient from 30% of MeOH to 100% MeOH V/V). According to differences in composition monitored by TLC, six fractions (G1–G6) were obtained. Fraction G2 (61 mg) was fractionated on a Sephadex LH-20 column eluting with acetone, yielding two fractions. One fraction gave flavone 1 (30 mg).
2.3.1 7-O-{α-l-rhamnopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-β-d-glucopyranosyl} luteolin (1)
Yellow powder; m.p. = 230 °C; [α]20D = –0.56 (MeOH, C = 1); UV λ MeOHmax nm 254, 351; IR γ KBrmax cm−1 3294 (OH), 3089, 2930, 2861, 1640 (C=O), 1558, 1369, 1072; for 1H and 13C NMR spectral data, see Table 1; ESI-MS C33H40O20.
1H and 13C NMR spectral data of compound 1 in DMSO-d6/TFAA (9:1). Chemical shifts are given in ppm; multiplicities and coupling constants J in Hz
| C/H | δC | δH | C/H | δC | δH |
| GENIN | Glc1 | ||||
| 2 | 164.7 | – | 1 | 98.5 | 5.22d (J = 7.5) |
| 3 | 103.3 | 6.70s | 2 | 82.7 | 3.58m |
| 4 | 181.8 | – | 3 | 76.5 | 3.24t (J = 8.8) |
| 5 | 161.2 | – | 4 | 69.7 | 3.19m |
| 6 | 99.8 | 6.51 (d, J = 1.5) | 5 | 75.5 | 3.66m |
| 7 | 162.9 | – | 6 | 65.9 | 3.89d (J = 11.1) |
| 8 | 95.1 | 6.79 (d, J = 1.5) | 3.50m | ||
| 9 | 157.2 | – | |||
| 10 | 105.5 | – | Glc2 | ||
| 1′ | 121.5 | – | 1 | 104.9 | 4.51d (J = 7.8) |
| 2′ | 113.8 | 7.42brs | 2 | 74.7 | 3.19m |
| 3′ | 145.7 | – | 3 | 77.1 | 3.18m |
| 4′ | 149.9 | – | 4 | 70.8 | 3.20m |
| 5′ | 116.3 | 6,93 (d, J = 8,0) | 5 | 77.1 | 3.18m |
| 6′ | 119.4 | 7.43 (brd, J = 8.0) | 6 | 60.8 | 3.56m |
| 3.49m | |||||
| Rham | |||||
| 1 | 100.4 | 4.58brs | |||
| 2 | 70.4 | 3.70brs | |||
| 3 | 70.8 | 3.52m | |||
| 4 | 72.3 | 3.19m | |||
| 5 | 68.4 | 3.46dq (J = 9.1;6.2) | |||
| 6 | 17.8 | 1.09d (J = 6.2) |
2.3.2 7-O-β-d-apiofuranosyl (1 → 2)-β-d-xylopyranosyl luteolin (2)
Yellow powder; m.p. = 203 °C; [α]20D = –45 (MeOH, C = 1); UV λ MeOHmax nm 254 , 351; 1H NMR spectral data (500.13 MHz, CD3OD): aglycone δ 6.49 (1H, J = 1.5 Hz, H-6), 6.77 (1H, d, J = 1.5 Hz, H-8), 6.94 (1H, d, J = 8.0 Hz, H-6′), 7.41 (1H, brs, H-2′), 7.43 (1H, brd, J = 8.0 Hz, H-6′); xylose δ 3.08 (1H, d, J = 11.0 Hz, H-5A), 3.22 (2H, m, H-2 and H-4), 3.30 (1H, m, H-3), 3.70 (1H, m, H-5B), 4.25 (1H, d, J = 7.5 Hz); apiose d 3.63 (2H, m, H-5), 3.79 (1H, d, J = 10.0 Hz, H-4A), 4.01 (1H, d, J = 3.0 Hz, H-2), 4.15 (1H, d, J = 10.0 Hz, H-4B), 5.25 (1H, d, J = 3.0 Hz, H-1); 13C NMR spectral data (125.75 MHz, CD3OD): aglycone δ 95.93 (C-8), 100.82 (C-6), 104.10 (C-3), 107.06 (C-10), 114.21 (C-2′), 116.87 (C-5′), 120.57 (C-6′), 123.27 (C-1′), 147.19 (C-3′), 151.59 (C-4′), 158.88 (C-9), 162.93 (C-5), 164.42 (C-7), 166.89 (C-2), 183.97 (C-4); xylose: δ 66.87 (C-5), 70.95 (C-4), 78.20 (C-3),78.79 (C-2), 100.87 (C-1); apiose: δ 65.86 (C-5), 75.44 (C-4), 77.88 (C-2), 80.68 (C-3), 111.08 (C-1); ESI-MS C25H26O14.
2.3.3 7-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl luteolin (3)
13C NMR spectral data (125.75 MHz, CD3OD): aglycone δ 95.88 (C-8), 100.48 (C-6), 104.17 (C-3), 107.07 (C-10), 114.29 (C-2′), 116.84 (C-5′), 120.55 (C-6′), 123.46 (C-1′), 147.08 (C-3′), 151.28 (C-4′), 158.88 (C-9), 162.92 (C-5), 164.20 (C-7), 166.83 (C-2), 183.97 (C-4); glucose: δ 67.30 (C-6), 70.96 (C-4), 74.02 (C-2), 78.62 (C-5), 79.07 (C-3), 100.87 (C-1); rhamnose: δ 18.25 (C-6), 70.05 (C-5), 71.17 (C-2), 72.21 (C-3), 72.55 (C-4), 100.48 (C-1); ESI-MS: m/z 629 [M–H]– corresponding to the molecular formula C27H34O17.
3 Results
The extraction was realized from the decoction by repeated column chromatography yielding three pure compounds.
Compound 1 was obtained as yellow powder and gave a yellow orange color by spraying Neu's reagent (1% diphenylboric ethanolamine complex in MeOH).
The ESI MS gave a molecular peak m/z 755 [M–H]– corresponding to the molecular formula C33H40O20. Other important fragments in the spectrum were at m/z 609 [M–146–H]– (loss of a deoxyhexose moiety), m/z 593 [M–162–H]– (loss of a hexose moiety), m/z 447 [M–162–146–H]– (loss of a hexose and deoxyhexose moieties), m/z 285 [M–162–146–162–H]– (loss of two hexoses and one deoxyhexose moieties). These results indicated the presence of three sugars, with the inner hexose directly linked to the aglycone and bearing two terminal sugars (a hexose and a deoxyhexose).
The downfield shifts observed for the C-6 (99.8 ppm) and C-8 (95.1 ppm) resonances of luteolin suggested that the sugar chain is linked to C-7 of the aglycone. The HMBC spectrum showed a correlation between C-7 of the luteolin and the anomeric proton of the inner glucose confirming that the glucose is directly linked to luteolin at this position. The 13C NMR signal at δ 82.7 ppm attributed to C-2 of the inner glucose suggested a glucosyl-(1 → 2) glucosyl moiety (as in sophorosyl) [1]. The HMBC spectrum exhibited a correlation between C-2 of the inner glucose and the anomeric proton of the terminal glucose. The HMBC spectrum also showed a correlation between C-6 of the inner glucose (δ 65.9 ppm) and the anomeric proton of the terminal rhamnose, in agreement with a rhamnosyl (1 → 6) glucosyl moiety (as in rutinosyl) [1]. This sugar chain has already been described for a kaempferol glycoside [2,3]. The NMR spectral data are in good agreement with those previously reported. From the above spectral evidence, the structure of compound 1 was established as 7-O-{α-l-rhamnopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-β-d-glucopyranosyl} luteolin, a new compound (Fig. 1).

Structure of the compound 1.
Compound 2 gave a yellow orange color with Neu's reagent. The aglycone was identified as luteolin by comparison with literature data [1]. Negative ESI mass spectrum gave a peak at m/z = 549 [M–H]– corresponding to the molecular formula C25H26O14. Other important fragments were observed at m/z 417 [M–162–H]– and at m/z 285 [M – 162 – 162 – H]–. These results were confirmed by 13C NMR spectral data. Downfield shifts observed for C-6 and C-8 resonances were indicative of the C-7 substitution. The presence of the disaccharide β-d-xylose (2 → 1) apiose linked at the C-7 of the aglycone was deduced by comparison with previous reported 13C NMR data [4] for such sugar linkage and confirmed by the analysis of the COSY spectrum.
The structure of 2 was elucidated as 7-O-β-d-apiofuranosyl (1 → 2)-β-d-xylopyranosyl luteolin 2. The campanoside acetate (in position 3 of apiose) of the structure 2 was previously reported in Campanula patula [5], but flavone 2 is a new compound.
All carbon signals of the compound 3 were assigned by comparison with the previously reported 13C NMR [6,7]. The structure of 3 was elucidated as: 7-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl luteolin. This flavone is described for the first time in the plant (Fig. 2).
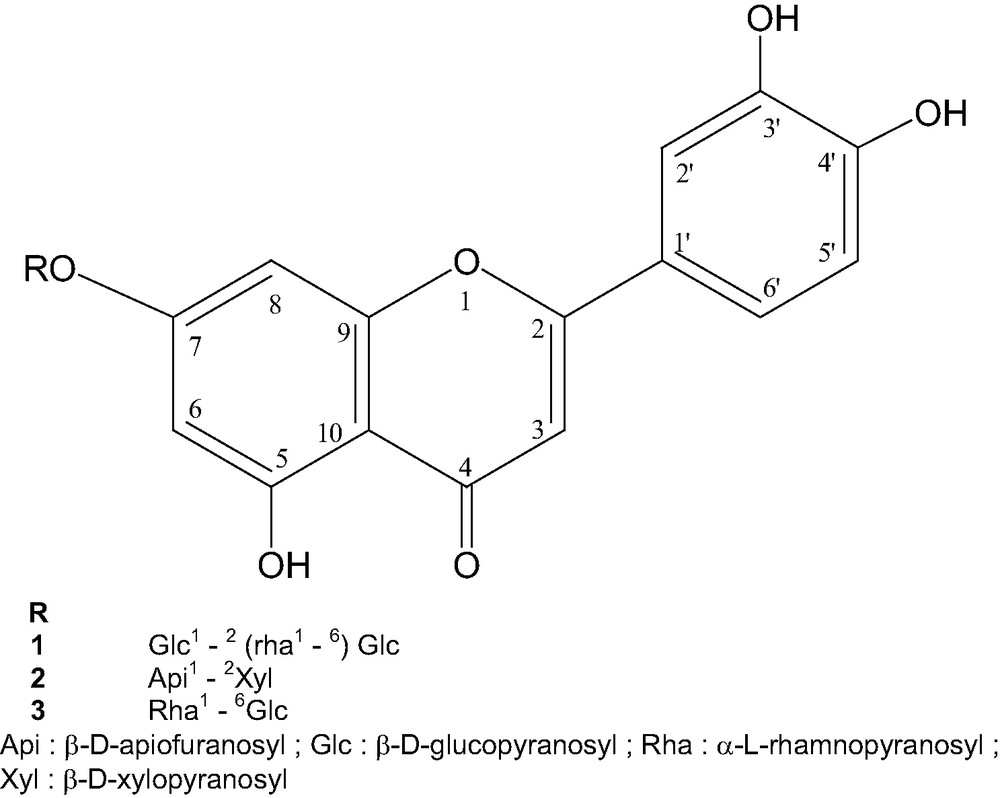
Structure of flavone glycosides.
4 Conclusion
Three flavones are described for the first time in this plant and two of them are new compounds. This work is a contribution to the knowledge of the chemistry of S. vogelii.
It would be interesting to study if these flavonoids participate to the activity of the plant.


