1 Introduction
Recently, Bernal et al. [1] announced the discovery of a hydronium di-cation of composition H14O62+ that had been trapped in a crystalline lattice by a cucurbituril molecule. The cucurbituril molecule has an upper and a lower crown of six carbonyl moieties which act as a perfect trap for the H14O62+ cation—one of each trapped on top and bottom of the molecule. In view of the fact that such a development was totally un-precedented, we decided to search for the existing literature in order to ascertain whether or not there were additional examples of cyclic hydronium cations which had also gone un-noticed. In fact, the search produced an identical cation of composition H14O62+, which appears in the CSD compilation1 as BIKVIA10 [2]. For more details, see a review of the stereochemistry of hydronium ions [3].
2 Discussion
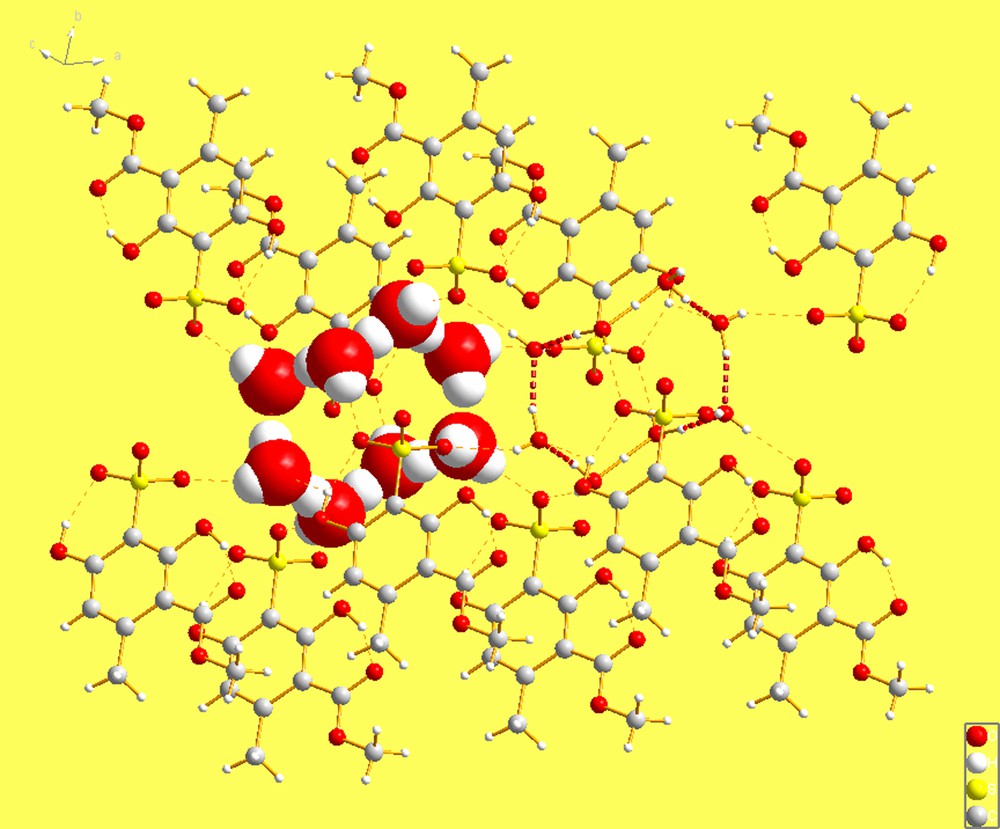
A more recent search has revealed the presence of another cyclic hydronium di-cation of composition H18O82+, which has been in the literature since 1987 [4] and has also gone un-noticed till now. This remarkable new species can be viewed as a ring of eight waters which have been protonated twice at positions trans to each other, forming a ring not too different in geometry from that in cyclooctane. Fig. 1 shows the di-cation and its bond lengths. Note that it is located at an inversion center of the space group P(; No. 2).

The stereochemistry of the cation together with its dimensions.
As in the case of the previous cyclic species (H14O62+) the new cation is trapped in the lattice by an organic species, in this case bearing sulfonates and hydroxyl functional groups. The cation is trapped by hydrogen bonds, as shown in Fig. 2. An interesting additional comment is that the same organic species can also trap a classically known hydronium mono-cation; namely H9O41+, which is the monomeric species of the, formally, dimeric species we describe herein. However, as shown in Fig. 3, H9O41+ discovered by Hanson, and described in the same paper [4], is a branched, star-like species which cannot be dimerized into the (H18O82+) di-cation without a drastic rearrangement. The fact that both of these species can be trapped by the same organic molecule is, in itself, a noteworthy observation, even though the conditions for their crystallization differ2 [4].
Dr. A.W. Hanson was notified by us of our discovery of his paper and was asked to join us in this report as a co-author. He graciously declined to join us; but has a copy of the manuscript. He may be contacted at his e-mail address AAlHanson@aol.com. A search in SciFinder Scholar reveals the existence of this hydronium di-cation only in Ref. [4]. See footnote 2 also.
1 CSD = Cambridge Structural Database, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, Tel.: +44 1223 336408. Available from http://wwsw.ccdc.cam.ac.uk. Released by Wavefunction, Inc., 18401 von Karman Ave., Suite 370, Irvine CA 92612. Tel.: +949 955 2120; fax: +949 955 2118. http://www.wavefun.com. Release of November 2005.
2 A search of SciFinder (Version of 2005) reveals no entries of any kind for Wolfgang Maass for the years 1986–1988 which were the years when he was, supposedly, going to publish the details of his method of obtaining the two crystalline forms containing the H9O41+ and the H18O82+ cations described by Hanson in Ref. [4].