1 Introduction
One of the features of the isoindole system is its ability for the isoindole–isoindoline isomery, which was first studied by Veber and Lwowski [1]. Isoindole form was calculated to be more favorable, but both are thermodynamically close. Therefore, for simple isoindoles it is possible to study the isomers in equilibrium with UV and NMR spectroscopy [2–9]. It is to notice that only one example of separation of each isomeric form in individual state is known in literature [10].
In another point of view, these products present often interesting biological properties and some closely related compounds [i.e. RN: 374703-27-2 and 866817-51-8] are included in recent screening libraries.
Demonstration of the presence of isomeric forms could also be expected for annelated isoindoles, but to the best of our knowledge no example was yet reported.
The difficulty to separate both isoindole and isoindoline forms is the crucial point of this research and we will afford here some new insight into it.
2 Results and discussion
2-(Bromomethyl)benzonitrile is known to react in solid state when heated with 2-aminothiophene-3-carboxylic acid esters derivatives yielding thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(10H)-one 1a, b [11,12]. This thermodynamically controlled reaction takes place when starting compounds are mixed with only a drop of DMF. According to the NMR data, only the isoindoline form 1a, b is present in CF3COOD solution.
However, when this reaction is carried out in other conditions, a mixture of isoindoline and isoindole forms was obtained (Scheme 1).
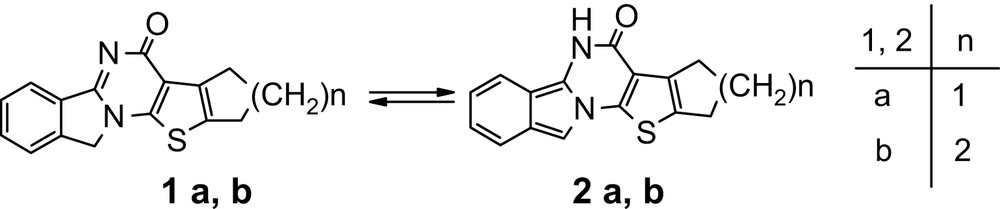
Thus, heating of 2-(bromomethyl)benzonitrile and 2-aminothiophene-3-carboxylic acid esters in excess of DMF leads to the formation of a mixture of compounds 1a, b and 2a, b, and their ratio in reaction mixture was 60:40, compounds 2a, b being formed under kinetic control. Moreover, heating the isoindole form in solution gives back the isoindoline isomer.
Compounds 1a, b and 2a, b were separated from the mixture by crystallization in DMF, taking into account the fact that isoindole products are less soluble than isoindoline ones. The structure of both isomers was confirmed by NMR, IR spectrometry and by the products of their reactions with maleinimide [13,14].
It is well known that the methylene groups in the pyrrolidine ring of the isoindoline isomers react with aldehydes in presence of sodium methoxide to yield benzylidene derivatives. So we decided to use also compounds 2a, b in this reaction and try to modify the α-position of isoindole isomers in other conditions.
The condensation of isoindoline compounds 1a, b with aromatic aldehydes was performed in MeOH in the presence of MeONa under reflux for a few hours, yielding 68–75% of compounds 5a–t (Scheme 2).
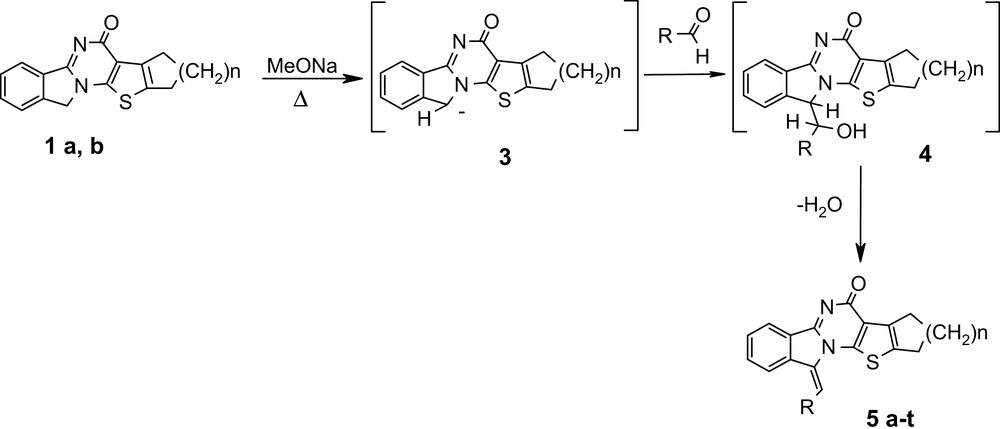
On the other hand, compounds 2 with isoindole structure were dissolved in boiling DMF with the same aldehyde, yielding also 82–97% of benzylidene compounds 5 a–t (Scheme 3).
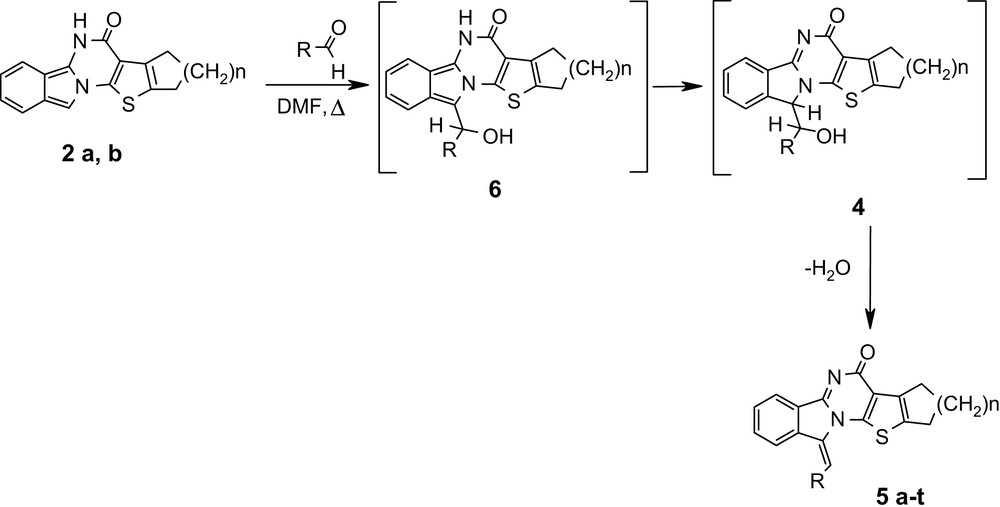
It is assumed that in both cases the aldol intermediates 4 are formed. However, for compounds 1a, b the reaction must begin with a α-pyrrole deprotonation (like in the Knoevenagel condensation). On the contrary, reaction of compounds 2a, b starts with an electrophilic attack on the α-pyrrole carbon atom (like in the Erhlich reaction). We guess that the formation of carbanion in this case is not possible, due to the presence of a 10p-electron fragment in the starting compound.
The benzilidene compounds 5 are obtained as a unique isomer, certainly the thermodynamic one E due to the rather hard reaction conditions.
Moreover, the reaction of isoindoline derivatives 1a, b with aldehydes in DMF did not lead to the formation of benzylidene derivatives, the starting compounds being recovered unchanged form the reaction mixture. These facts reinforce our assumption regarding the reaction mechanism.
Besides, the condensation of aliphatic aldehydes, namely propional and butanal, with compounds 1a, b or 2a, b leads only to tar formation, and no pure products were isolated. However, in the LCMS spectra of the reaction mixture, we observed peaks corresponding to the benzylidene compounds 5.
In conclusion, new benzylidene thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(10H)-one derivatives were synthesized starting separately from the isomeric compounds 1a, b and 2a, b. It was found that compounds 1a, b react with aldehydes only in the presence of sodium methoxide. The structure of the formed benzylidene derivatives was confirmed by NMR, UV spectroscopy (Table 1) and mass spectra.
Substitution scheme and some UV spectra in acetonitrile of compounds 5a
| 5 | R | n | λmax, nm | log ɛ | 5 | R | n | λmax, nm | log ɛ |
| a | 2 | 370.33 | 3.65 | b | 2 | 410.02 | 4.45 | ||
| c | 2 | 426.57 | 4.64 | d | 2 | 387.97 | 4.48 | ||
| e | 2 | 269.53 | 4.82 | f | 2 | 301.03 | 4.41 | ||
| g | 2 | 292.84 | 4.54 | h | 2 | 384.19 | 3.86 | ||
| i | 2 | 393.05 | 4.15 | j | 2 | 401.99 | 4.61 | ||
| k | 2 | 382.3 | 4.37 | l | 2 | 377.89 | 4.23 | ||
| m | 1 | 304.18 | 4.85 | n | 1 | – | – | ||
| o | 1 | – | – | p | 1 | – | – | ||
| q | 1 | – | – | r | 1 | – | – | ||
| s | 1 | – | – | t | 1 | – | – |
a Compounds 5n–t are insoluble in acetonitrile.
3 Experimental
Chemical ionization mass spectra were recorded on a Nermag R10 13C and NMR spectra on a Varian Mercury 400 (400 MHz) (1H: 400 MHz, 13C: 100 MHz) in DMSO-d6 and CF3COOD; δ values are given in ppm. UV–visible spectra were registered in acetonitrile with a PerkinElmer Lambda-19 spectrophotometer equipped with a 60-mm integration sphere for solid measurements. Elemental analyses were determined with a Carlo Erba Strumentazione apparatus.
3.1 General procedure for the preparation 1a, b or 2a, b
2-(Bromomethyl)benzonitrile (0.01 mmol) and the corresponding 2-aminothiophene-3-carboxylic acid esters (0.015 mmol) in dry DMF (2 ml) were heated under reflux for 10 min. After cooling, the product was collected by filtration, and recrystallized from DMF. Compounds 1a, b have poor solubility in hot DMF, whereas compounds 2a, b are soluble under these conditions.
We notice that in 1H NMR the NH proton of 2a, b is not recorded due to the exchange with the water traces in DMSO-d6.
3.1.1 8,9-Dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(12H)-one (1a)
Mp 332 °C; C16H12N2OS, M is 280.34. Analysis (calcd, found)% C (68.55, 68.61); H (4.31, 4.39); N (9.99, 10.03); [MH]+ 281. 1H NMR δ (DMSO-d6) 2.41–2.446 (m, 2H); 2.89–2.99 (m, 4H); 5.63 (s, 2H); 7.68 (t, 1H, J = 7.8 Hz); 7.78–7.87 (m, 2H); 8.24 (d, 1H, J = 8.0 Hz).
3.1.2 7,8,9,10-Tetrahydro [1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (1b)
Mp 328 °C; C17H14N2OS, M is 294.37. Analysis (calcd, found)% C (69.36, 69.65); H (4.79, 4.90); N (9.52, 9.60); [MH]+ 295. 1H NMR δ (DMSO-d6) 1.86–1.91 (m, 4H); 2.86–2.88 (m, 2H); 2.96–2.98 (m, 2H); 5.61 (s, 2H); 7.723 (t, 1H, J = 7.8 Hz); 7.84–7.91 (m, 2H); 8.36 (d, 1H, J = 8.0 Hz).
3.1.3 8,9-Dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(5H)-one (2a)
Mp 318 °C; C16H12N2OS, M is 280.34. Analysis (calcd, found)% C (68.55, 68.58); H (4.31, 4.34); N (9.99, 10.10); [MH]+ 281. 1H NMR δ (DMSO-d6) 2.40–2.44 (m, 2H); 2.90–2.98 (m, 4H); 6.63 (s, 1H); 7.54–7.65 (m, 3H,); 7.83 (d, 1H, J = 7.2 Hz).
3.1.4 7,8,9,10-Tetrahydro[1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(5H)-one (2b)
Mp 315 °C; C17H14N2OS, M is 294.37. Analysis (calcd, found)% C (69.36, 69.45); H (4.79, 4.83); N (9.52, 9.64); [MH]+ 295. 1H NMR δ (DMSO-d6) 1.80–1.86 (m, 4H); 2.74–2.76 (m, 2H); 2.91–2.95 (m, 2H); 6.63 (s, 2H); 7.55–7.65 (m, 3H); 8.36 (d, 1H, J = 7.6 Hz).
3.2 General procedure 1 for the preparation of 5a–t from 1a, b
Compound 1a or 1b (0.005 mmol) was suspended in a sodium methoxide solution (0,02 mmol sodium in 5 ml abs. methanol) and heated under reflux for 10 min. Then the corresponding aldehyde was added (0.008 mmol). The mixture was heated under reflux for 20 min. After cooling, the product was separated by filtration, and recrystallized from DMF.
3.3 General procedure 2 for the preparation of 5a–t from 2a, b
Compound 2a or 2b (0.005 mmol) was suspended in DMF with (0.01 mmol) of aldehydes and heated under reflux for 10 min. After cooling, the product was recovered by filtration, and crystallized in DMF. The respective yields of both processes are indicated hereafter in the form “Yields (1)/(2)%”.
3.3.1 13-[(E)-(5-Bromo-2-hydroxyphenyl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(13H)-one (5a)
Mp 356 °C; C24H17BrN2O2S, M 477.38. Analysis (calcd, found)% C (60.38, 60.47); H (3.59, 3.72); N (5.87, 6.03); [MH]+ 478. Yields (1)/(2)%: 68/82. 1H NMR δ (CF3COOD) 2.11–2.19 (m, 4H); 3.02–3.11 (m, 2H); 3.21–3.26 (m, 2H); 7.56 (d, 1H, J = 8.8 Hz); 7.67–7.72 (m, 1H); 7.91–8.05 (m, 5H); 8.22 (s, 1H); 8.45–8.48 (m, 1H).
3.3.2 13-[(E)-2-Benzofuranylmethylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6]pyrimido [2,1-a]isoindol-6(13H)-one (5b)
Mp 344 °C; [MH]+423. Yields (1)/(2)%: 73/85. 1H NMR δ (CF3COOD) 2.09–2.19 (m, 4H); 3.08–3.11 (m, 2H); 3.21–3.28 (m, 2H); 7.52 (t, 1H, J = 7.4 Hz); 7.70 (t, 1H); 7.73 (s, 1H); 7.78 (d, 1H, J = 7.2 Hz); 7.86 (d, 1H, J = 7.6 Hz); 8.01 (t, 1H, J = 7.8 Hz); 8.24 (t, 1H); 8.25 (s, 1H); 8.48 (d, 1H, J = 8.0 Hz); 9.53 (d, 1H, J = 8.4 Hz); 13C NMR δ (CF3COOD) 11.28; 20.86; 21.71; 24.07; 24.69; 54.29; 116.28; 121.58; 121.92; 122.61; 122.72; 123.24; 124.91; 126.28; 127.82; 129.84; 131.72; 132.22; 133.49; 134.23; 137.32; 138.18; 144.61; 147.77; 149.19; 150.48; 157.44; 157.72.
3.3.3 13-[(E)-(3,6-Dimethyl-2-benzofuranyl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno [3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (5c)
Mp 349 °C; [MH]+451. Yields (1)/(2)%: 70/83. 1H NMR δ (CF3COOD) 2.10–2.22 (m, 4H); 2.66 (s, 3H, CH3); 2.74 (s, 3H, CH3); 3.09–3.14 (m, 2H); 3.25–3.28 (m, 2H); 7.37 (d, 1H, J = 8.4 Hz); 7.51 (s,1H); 7.7 (d,1H, J = 7.6 Hz); 7.98 (t, 1H, J = 7.6 Hz); 8.21 (t, 1H, J = 7.6 Hz); 8.24 (s, 1H); 8.44 (d, 1H, J = 8.0 Hz); 9.51 (d, 1H, J = 8.4 Hz); 13C NMR δ (CF3COOD) 8.00; 20.50; 20.80; 21.65; 24.01; 24.68; 54.26; 111.10; 113.76; 120.67; 121.21; 122.56; 122.88; 125.94; 126.31; 129.77; 131.05; 133.55; 133.64; 134.22; 136.84; 137.74; 143.17; 144.29; 144.44; 148.97; 157.32; 157.39.
3.3.4 7,8,9,10-Tetrahydro-13-[(E)-(4-methoxyphenyl)methylidene]-[1]benzothieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(13H)-one (5d)
Mp 329 °C; [MH]+413. Yields (1)/(2)%: 76/95. 1H NMR δ (CF3COOD) 2.12–218 (m, 4H); 3.06–3.09 (m, 2H); 3.24–3.27 (m, 2H); 4.16 (s, 3H, CH3); 7.35 (d, 2H, J = 6.8 Hz); 7.81 (d, 2H, J = 7.2 Hz); 7.90–7.95 (m, 2H); 8.12–8.16 (m, 1H); 8.42–8.45 (m, 1H); 8.52 (s, 1H).
3.3.5 7,8,9,10-Tetrahydro-13-[(E)-phenylmethylidene]-[1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (5e)
Mp 332 °C; C24H18N2OS, M 382.49. Analysis (calcd, found)% C (75.37, 75.43); H (4.74, 4.76); N (7.32, 7.36); [MH]+ 383. Yields (1)/(2)%: 75/97. 1H NMR δ (CF3COOD) 2.11–2.18 (m, 4H); 3.09–3.12 (m, 2H); 3.25–3.29 (m, 2H); 7.73–7.81 (m, 5H); 7.87–7.99 (m, 3H); 8.45 (d, 1H, J = 7.6 Hz); 8.61 (s, 1H).
3.3.6 13-[(E)-(2-Fluorophenyl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6]pyrimido [2,1-a]isoindol-6(13H)-one (5f)
Mp 336 °C; C24H17FN2OS, M 400.48. Analysis (calcd, found)% C (71.98, 72.03); H (4.28, 4.30); N (6.99, 7.03); [MH]+ 401. Yields (1)/(2)%: 69/90. 1H NMR δ (CF3COOD) 2.25–2.29 (m, 4H); 3.20–3.24 (m, 2H); 3.40–3.43 (m, 2H); 7.59 (t, 1H, J = 9.8 Hz); 7.65 (t, 1H, J = 7.2 Hz); 7.90–7.98 (m, 2H); 8.01–8.10 (m, 3H); 8.57–8.61 (m, 2H).
3.3.7 13-[(E)-(4-Fluorophenyl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6]pyrimido [2,1-a]isoindol-6(13H)-one (5g)
Mp 338 °C; [MH]+ 401. Yields (1)/(2)%: 73/92. 1H NMR δ (CF3COOD) 2.20–2.31 (m, 4H); 3.21–3.24 (m, 2H); 3.40–3.42 (m, 2H); 7.55–7.60 (m, 2H); 7.90–7.95 (m, 2H); 8.04–8.11 (m, 3H); 8.59 (d, 1H, J = 8.0 Hz); 8.67 (s, 1H).
3.3.8 13-[(E)-(3,4-Dimethoxyphenyl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(13H)-one (5h).
Mp 325 °C; [MH]+ 443. Yields (1)/(2)%: 68/88. 1H NMR δ (CF3COOD) 2.52–2.59 (m, 4H); 3.45–3.49 (m, 2H); 3.62–3.68 (m, 2H); 4.48 (s, 3H, CH3); 4.56 (s, 3H, CH3); 7.70 (d, 1H, J = 8.4 Hz); 7.79 (s, 1H); 7.93 (d, 1H, J = 8.4 Hz); 8.31–8.38 (m, 2H); 8.56–8.61 (m, 1H); 8.82–8.87 (m, 1H); 8.90 (s, 1H).
3.3.9 13-[(E)-(5-Bromo-3,4-dihydro-6-methoxy-2H-1-benzopyran-8-yl)methylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (5i)
Mp 347 °C; C28H23BrN2O3S, M 547.48. Analysis (calcd, found)% C (61.43, 61.41); H (4.23, 4.25); N (5.12, 5.17); [MH]+ 548. Yields (1)/(2)%: 73/95. 1H NMR δ (CF3COOD) 2.09–2.18 (m, 4H); 2.22–2.28 (m, 2H); 3.02–3.10 (m, 4H); 3.22–3.25 (m, 2H); 4.07 (s, 3H, CH3); 4.36–4.40 (m, 2H); 7.40 (s, 1H); 7.91–7.96 (2H); 8.11–8.14 (m, 1H); 8.40–8.48 (m, 1H); 8.50 (s, 1H).
3.3.10 7,8,9,10-Tetrahydro-13-[(E,2E)-3-phenyl-2-propenylidene]-[1]benzothieno[3′,2′:5,6]pyrimido [2,1-a]isoindol-6(13H)-one (5j)
Mp 342 °C; [MH]+ 409. Yields (1)/(2)%: 73/90. 1H NMR δ (CF3COOD) 2.09–2.18 (m, 4H); 3.09–3.11 (m, 2H); 3.21–3.25 (m, 2H); 7.52–7.59 (m, 3H); 7.63 (d, 1H, J = 14.8); 7.74–7.79 (m, 2H); 7.96 (t, 1H, J = 7.6 Hz); 8.04–8.10 (m, 1H); 8.17–8.23 (m, 2H); 8.46 (d, 1H, J = 8.0 Hz); 8.52 (d, 1H, J = 7.6 Hz); C26H20N2OS, M is 408.53. Analysis (calcd, found)% C (76.44, 76.49); H (4.93, 4.98); N (6.86, 6.70).
3.3.11 13-[(E)-2-Furanylmethylidene]-7,8,9,10-tetrahydro-[1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (5k)
Mp 327 °C; C22H16N2O2S, M 372.45. Analysis (calcd, found)% C (70.95, 70.98); H (4.33, 4.38); N (7.52, 7.58); [MH]+ 373. Yields (1)/(2)%: 71/95. 1H NMR δ (CF3COOD) 1.95–2.19 (m, 4H); 2.95–3.12 (m, 2H); 3.21–3.29 (m, 2H); 6.92–6.94 (m, 1H); 7.43 (d, 1H, J = 4.0 Hz); 7.95 (t, 1H, J = 7.6 Hz); 8.09–8.11 (m, 1H); 8.13 (s, 1H); 8.43 (d, 1H, J = 8.0 Hz); 9.31 (d, 1H, J = 8.4 Hz).
3.3.12 7,8,9,10-Tetrahydro-13-[(E)-2-thienylmethylidene]-[1]benzothieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(13H)-one (5l)
Mp 333 °C; C22H16N2OS2, M 388.51. Analysis (calcd, found)% C (68.01, 68.03); H (4.15, 4.18); N (7.21, 7.25); [MH]+ 389. Yields (1)/(2)%: 69/92. 1H NMR δ (CF3COOD) 2.09–2.13 (m, 4H); 3.04–3.09 (m, 2H); 3.19–3.23 (m, 2H); 7.43 (t, 1H, J = 4.4); 7.85 (d, 1H, J = 3.6); 7.93 (t, 1H, J = 7.8); 7.98 (d, 1H, J = 5.2); 8.02 (t, 1H, J = 7.8); 8.43 (d, 1H, J = 8.0); 8.54 (s, 1H); 8.69 (d, 1H, J = 8.0).
3.3.13 8,9-Dihydro-12-[(E)-phenylmethylidene]-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(12H)-one (5m)
Mp 325 °C; Yields (1)/(2)%: 74/92. 1H NMR δ (CF3COOD) 2.80–2.86 (m, 2H); 3.29–3.41 (m, 4H); 7.70–7.81 (m, 5H); 8.82–8.91 (m, 3H); 8.42 (d, 1H, J = 7.6 Hz); 8.61 (s, 1H); 13C NMR δ (CF3COOD) 27.83; 27.87; 28.48; 54.26; 122.62; 123.36; 124.32; 128.90; 129.39; 130.18; 131.51; 132.03; 132.50; 133.81; 135.18; 137.13; 142.97; 143.96; 148.69; 152.02; 157.31.
3.3.14 8,9-Dihydro-12-[(E)-4-pyridinylmethylidene]-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5n)
Mp 330 °C; C22H15N3OS, M 369.45. Analysis (calcd, found)% C (71.52, 72.54); H (4.09, 4.12); N (11.37, 11.43); [MH]+ 370. Yields (1)/(2)%: 71/89. 1H NMR δ (CF3COOD) 2.79–2.83 (m, 2H); 3.21–3.43 (m, 4H); 7.56 (d, 1H, J = 8.4 Hz); 7.89 (t, 1H, J = 7.6 Hz); 8.00 (t, 1H, J = 7.6); 8.42 (s, 1H); 8.49 (d, 1H, J = 8.0 Hz); 8.56 (d, 2H, J = 6.4 Hz); 9.21 (d, 2H, J = 4.8 Hz).
3.3.15 12-[(E)-(4-Chlorophenyl)methylidene]-8,9-dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5o)
Mp 336 °C; Yields (1)/(2)%: 71/85. 1H NMR δ (CF3COOD) 2.80–2.85 (m, 2H); 3.28–3.37 (m, 4H); 7.69–7.73 (m, 4H); 7.89–7.92 (m, 3H); 8.44 (d, 1H, J = 6.8 Hz); 8.51 (s, 1H).
3.3.16 12-[(E)-(2-Fluorophenyl)methylidene]-8,9-dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5p)
Mp 331 °C; C23H15FN2OS, M 386.45. Analysis (calcd, found)% C (71.49, 71.51); H (3.91, 3.93); N (7.25, 7.27); [MH]+ 387. Yields (1)/(2)%: 75/93. 1H NMR δ (CF3COOD) 2.67–2.81 (m, 2H); 3.25–3.36 (m, 4H); 7.42 (t, 1H, J = 9.0 Hz); 7.43 (t, 1H, J = 7.6 Hz); 7.73–7.79 (m, 2H); 7.85–7.98 (m, 3H); 8.40–8.43 (m, 1H); 8.44 (s, 1H).
3.3.17 12-[(E)-(3-Fluorophenyl)methylidene]-8,9-dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5q)
Mp 348 °C; C23H15FN2OS, M 386.45. Analysis (calcd, found)% C (71.49, 71.54); H (3.91, 3.90); N (7.25, 7.30); [MH]+ 387. Yields (1)/(2)%: 70/96. 1H NMR δ (CF3COOD) 2.83–2.87 (m, 2H); 3.29–3.38 (m, 4H); 7.43–7.49 (m, 2H); 7.55 (d, 1H, J = 7.6 Hz); 7.72–7.75 (m, 1H); 7.83 (d, 1H, J = 7.6 Hz); 7.90–7.99 (m, 2H); 8.44 (d, 1H, J = 8.4 Hz); 8.54 (s, 1H).
3.3.18 12-[(E)-(4-Fluorophenyl)methylidene]-8,9-dihydro-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5r)
Mp 352 °C; C23H15FN2OS, M 386.45. Analysis (calcd, found)% C (71.49, 71.47); H (3.91, 3.95); N (7.25, 7.29); [MH]+ 387. Yields (1)/(2)%: 69/89. 1H NMR δ (CF3COOD) 2.78–2.81 (m, 2H); 3.28–3.35 (m, 4H); 7.36–7.40 (m, 2H); 7.74–7.77 (m, 2H); 7.87–7.94 (m, 3H); 8.40 (d, 1H, J = 6.8 Hz); 8.51 (s, 1H).
3.3.19 8,9-Dihydro-12-[(E,2E)-3-phenyl-2-propenylidene]-7H-cyclopenta[4′,5′]thieno[3′,2′:5,6] pyrimido[2,1-a]isoindol-6(12H)-one (5s)
Mp 350 °C; C25H18FN2OS, M 394.50. Analysis (calcd, found)% C (76.12, 76.15); H (4.60, 4.65); N (7.10, 7.12); [MH]+ 395. Yields (1)/(2)%: 72/93. 1H NMR δ (CF3COOD) 2.80–2.85 (m, 2H); 3.29–3.35 (m, 4H); 7.54–7.61 (m, 4H); 7.65 (d, 1H, J = 10.0); 7.77–7.81 (m, 2H); 8.00 (t, 1H); 8.06–8.11 (m, 1H); 8.212 (t, 1H); 8.26 (d, 1H, J = 11.2 Hz); 8.48 (d, 1H, J = 8.0 Hz); 8.55 (d, 1H, J = 8.0 Hz).
3.3.20 (12E)-12-(2,3-Dihydro-1,4-benzodioxin-6-ylmethylene)-8,9-dihydro-7H-cyclopenta[4′,5′] thieno[3′,2′:5,6]pyrimido[2,1-a]isoindol-6(12H)-one (5t)
Mp 347 °C; C25H18N2O3S, M 426.50. [MH]+ 427. Yields (1)/(2)%: 70/96. 1H NMR δ (CF3COOD) 2.74–2.80 (m, 2H); 3.15–3.30 (m, 4H); 7.20–7.22 (d, 1H, J = 8.4 Hz); 7.32–7.34 (d, 1H, J = 8.8 Hz); 7.37 (s, 1H); 7.62–7.63 (m, 1H); 8.00–8.02 (m, 1H); 8.13–8.15 (d, 1H); 8.36–8.41 (d, 1H); 8.46 (s, 1H).


