1 Introduction
The rational design and synthesis of metal oxide-based organic–inorganic hybrid materials with a two-dimensional or three-dimensional structure are receiving increasing interests in solid-state chemistry owing to their fascinating properties and great potential applications in many fields (such as catalysis, material science and medicine) as well as their unusual topological properties [1–4].
Many reduced molybdenum phosphates with transition-metal complexes have been synthesized and characterized in the past decade. These compounds include one-dimensional (1D) polymers, two-dimensional (2D) layer structures and three-dimensional (3D) microporous solids containing the Na[Mo6P4]2 [5], Fe[Mo6P4]2 [6], Cr[Mo6P4]2 [7], Cd[Mo6P4]2 [8], Co[Mo6P4]2 [9], and Ni[Mo6P4]2 [10] structure units. Only a few molybdenum phosphates containing the manganese element, such as (NH3CH2CH2NH3)10(H3O)3(H5O2)Na2[MnMo12O24(OH)6(PO4)4(PO3OH)4][MnMo12O24(OH)6(PO4)6(PO3OH)2]·9H2O and Na2[{Mn(phen)2(H2O)}{Mn(phen)2}3{MnMo12O24(HPO4)6(PO4)2(OH)6}]·4H2O have been reported [11]. Two [Mo6O12(OH)3(PO4)2(HPO4)2]7− units are bonded together by a manganese ion while several P–O groups are not protonated on account of coordination to a Na+ cation in the former compound. The latter compound may be considered to be based on {Mo6O12(HPO4)3(PO4)(OH)3} units bonded together with {Mn(phen)2} subunits.
Herein we wish to report the hydrothermal synthesis and structural characterization of a novel manganese molybdenum(V) phosphate, [Mn2Mo12O30(HPO4)8(H2O)]·(NH3CH2CH2NH3)6·3.5H2O (1). To our knowledge, the two-dimensional network structure which is constructed from molybdenum(V) phosphate interconnected only with manganese cations has not been reported up to date.
2 Experimental
2.1 Hydrothermal synthesis and characterization
Na2MoO4·2H2O (1.20 g, 4.96 mmol), MnSO4 (0.38 g, 2.50 mmol), ethylenediamine (0.40 ml, 3.03 mmol), H3PO4 (0.5 ml) and H2O (15 ml) were mixed and transferred into a 30-ml Teflon-lined stainless steel autoclave and heated at 160 °C for 6 days. Then, the autoclave was cooled to room temperature at 5 °C h−1. The black crystals of compound 1 were filtered off, washed with water and dried in a desiccator at ambient temperature (yield 37% based on Mo). Infrared spectrum was measured on a Nicolet AVATAR 360 FT-IR spectrometer on KBr pellets in the 4000–400 cm−1 region.
2.2 X-ray crystallography
A black single crystal with dimensions of 0.40 × 0.10 × 0.40 mm was mounted on a glass fibre. Data collection was performed on a Rigaku Weissenberg IP diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å). The structure was solved by the direct method using SHELXS-86 and refined by full-matrix least squares calculations using SHELXL-97 software. All non-hydrogen atoms were refined with anisotropic thermal parameters. Most of the hydrogen atoms were located in calculated positions and/or in the positions from difference Fourier map. The crystallographic data are given in Table 1. Selected bond lengths and angles are provided in Tables 2 and 3.
Crystallographic data for compound 1
| Formula | H77C12Mn2Mo12N12O66.5P8 |
| Fw | 2962.78 |
| Color | Black |
| Crystal system | Triclinic |
| Space group | |
| F (000) | 2878 |
| a (Å) | 12.006(2) |
| b (Å) | 14.582(3) |
| c (Å) | 21.270(4) |
| α (°) | 80.96(3) |
| β (°) | 83.24(3) |
| γ (°) | 76.33(3) |
| V (Å3) | 3560.8(1) |
| Z | 2 |
| Dcalc. (g cm−3) | 2.763 |
| μ (mm−1) | 2.691 |
| θ Range (°) | 3.02–27.48 |
| hmin→max | −15 → 15 |
| kmin→max | −18 → 18 |
| lmin→max | −27 → 26 |
| No. of unique data | 15 522 |
| No. of observed | 12 917 |
| Parameter | 1019 |
| GOF | 1.146 |
| R/Rw | 0.0604/0.1715 |
| Δρmax/Δρmin (e/Å3) | 2.409/(−1.662) |
Selected bond lengths (Å) of compound 1
| Bond | Distance (Å) | Bond | Distance (Å) | Bond | Distance (Å) |
| Mo(1)–O(28) | 1.683(8) | Mo(1)–O(49) | 1.935(8) | Mo(1)–O(50) | 1.970(8) |
| Mo(1)–O(32) | 2.033(8) | Mo(1)–O(21)#1 | 2.128(8) | Mo(1)–O(8) | 2.289(8) |
| Mo(1)–Mo(9) | 2.5994(16) | Mo(2)–O(2) | 1.685(8) | Mo(2)–O(58) | 1.948(8) |
| Mo(2)–O(48) | 1.978(7) | Mo(2)–O(43)#2 | 2.053(8) | Mo(2)–O(46)#2 | 2.084(8) |
| Mo(2)–O(4)#2 | 2.289(7) | Mo(2)–Mo(8) | 2.5884(15) | Mo(3)–O(42) | 1.694(9) |
| Mo(3)–O(57) | 1.945(8) | Mo(3)–O(54) | 1.969(8) | Mo(3)–O(38)#3 | 2.054(8) |
| Mo(3)–O(31) | 2.095(7) | Mo(3)–O(15) | 2.291(8) | Mo(3)–Mo(5) | 2.5870(16) |
| Mo(4)–O(1) | 1.690(9) | Mo(4)–O(56) | 1.961(8) | Mo(4)–O(59) | 1.973(7) |
| Mo(4)–O(25) | 2.060(8) | Mo(4)–O(46) | 2.075(8) | Mo(4)–O(4) | 2.263(8) |
| Mo(4)–Mo(10) | 2.6011(16) | Mo(5)–O(6) | 1.682(8) | Mo(5)–O(57) | 1.946(8) |
| Mo(5)–O(54) | 1.962(7) | Mo(5)–O(24) | 2.064(7) | Mo(5)–O(37)#1 | 2.100(8) |
| Mo(5)–O(44) | 2.284(8) | Mo(6)–O(41) | 1.688(8) | Mo(6)–O(39) | 1.947(8) |
| Mo(6)–O(55) | 1.980(8) | Mo(6)–O(45)#1 | 2.042(8) | Mo(6)–O(21) | 2.110(8) |
| Mo(6)–O(8)#1 | 2.282(8) | Mo(6)–Mo(12) | 2.6053(14) | Mo(7)–O(12) | 1.694(8) |
| Mo(7)–O(51) | 1.932(8) | Mo(7)–O(34) | 1.973(7) | Mo(7)–O(53) | 2.027(8) |
| Mo(7)–O(35) | 2.108(8) | Mo(7)–O(29)#2 | 2.343(7) | Mo(7)–Mo(11) | 2.5903(14) |
| Mo(8)–O(17) | 1.691(8) | Mo(8)–O(58) | 1.941(7) | Mo(8)–O(48) | 1.965(7) |
| Mo(8)–O(36) | 2.087(8) | Mo(8)–O(35) | 2.101(8) | Mo(8)–O(29)#2 | 2.241(7) |
| Mo(9)–O(23) | 1.691(8) | Mo(9)–O(49) | 1.941(8) | Mo(9)–O(50) | 1.973(8) |
| Mo(9)–O(47)#3 | 2.085(8) | Mo(9)–O(31) | 2.108(8) | Mo(9)–O(15) | 2.308(7) |
| Mo(10)–O(19) | 1.683(8) | Mo(10)–O(56) | 1.953(8) | Mo(10)–O(59) | 1.961(8) |
| Mo(10)–O(52)#2 | 2.065(8) | Mo(10)–O(30)#2 | 2.090(8) | Mo(10)–O(33) | 2.287(7) |
| Mo(11)–O(9) | 1.685(8) | Mo(11)–O(51) | 1.935(8) | Mo(11)–O(34) | 1.962(7) |
| Mo(11)–O(10) | 2.081(8) | Mo(11)–O(30) | 2.126(8) | Mo(11)–O(33)#2 | 2.286(7) |
| Mo(12)–O(20) | 1.684(8) | Mo(12)–O(39) | 1.939(8) | Mo(12)–O(55) | 1.970(7) |
| Mo(12)–O(26)#1 | 2.080(7) | Mo(12)–O(37) | 2.121(7) | Mo(12)–O(44)#1 | 2.233(8) |
| Mn(1)–O(34) | 2.187(7) | Mn(1)–O(59) | 2.207(7) | Mn(1)–O(48) | 2.234(7) |
| Mn(2)–O(55) | 2.192(7) | Mn(2)–O(50) | 2.214(8) | Mn(2)–O(54) | 2.217(8) |
| Mn(3)–O(62) | 2.103(9) | Mn(3)–O(18) | 2.162(9) | Mn(3)–O(11) | 2.170(9) |
| Mn(3)–O(5) | 2.194(10) | Mn(3)–O(1W) | 2.236(15) | P(1)–O(3) | 1.517(9) |
| P(1)–O(32) | 1.540(9) | P(1)–O(40) | 1.546(9) | P(1)–O(45) | 1.546(9) |
| P(2)–O(11) | 1.508(8) | P(2)–O(15) | 1.538(8) | P(2)–O(44) | 1.545(8) |
| P(2)–O(8) | 1.551(8) | P(3)–O(7) | 1.511(9) | P(3)–O(60) | 1.526(9) |
| P(3)–O(53) | 1.552(9) | P(3)–O(36) | 1.560(9) | P(4)–O(61) | 1.492(10) |
| P(4)–O(25) | 1.535(9) | P(4)–O(43) | 1.545(8) | P(4)–O(27) | 1.586(9) |
| P(5)–O(22) | 1.517(9) | P(5)–O(5) | 1.525(9) | P(5)–O(47) | 1.556(9) |
| P(5)–O(38) | 1.575(9) | P(6)–O(18) | 1.507(9) | P(6)–O(14) | 1.542(8) |
| P(6)–O(24) | 1.551(8) | P(6)–O(26) | 1.551(8) | P(7)–O(62) | 1.493(9) |
| P(7)–O(52) | 1.518(9) | P(7)–O(10) | 1.552(8) | P(7)–O(13) | 1.566(9) |
| P(8)–O(16) | 1.513(8) | P(8)–O(4) | 1.545(8) | P(8)–O(29) | 1.553(7) |
| P(8)–O(33) | 1.556(8) |
Selected bond angles (°) of compound 1
| Angle | (°) | Angle | (°) | Angle | (°) |
| O(28)–Mo(1)–O(49) | 106.6(4) | O(28)–Mo(1)–O(50) | 101.3(4) | O(49)–Mo(1)–O(50) | 95.2(3) |
| O(28)–Mo(1)–O(32) | 97.3(4) | O(49)–Mo(1)–O(32) | 86.8(4) | O(50)–Mo(1)–O(32) | 159.8(3) |
| O(28)–Mo(1)–Mo(9) | 100.9(3) | O(49)–Mo(1)–Mo(9) | 48.0(2) | O(50)–Mo(1)–Mo(9) | 48.8(2) |
| O(32)–Mo(1)–Mo(9) | 134.4(3) | O(21)#1–Mo(1)–Mo(9) | 134.8(2) | O(8)–Mo(1)–Mo(9) | 89.43(19) |
| O(2)–Mo(2)–O(58) | 105.0(4) | O(2)–Mo(2)–O(48) | 101.4(4) | O(58)–Mo(2)–O(48) | 95.6(3) |
| O(2)–Mo(2)–Mo(8) | 100.8(3) | O(58)–Mo(2)–Mo(8) | 48.2(2) | O(48)–Mo(2)–Mo(8) | 48.7(2) |
| O(42)–Mo(3)–O(31) | 98.5(4) | O(57)–Mo(3)–O(31) | 155.1(3) | O(54)–Mo(3)–O(31) | 84.8(3) |
| O(42)–Mo(3)–Mo(5) | 99.3(3) | O(57)–Mo(3)–Mo(5) | 48.3(2) | O(54)–Mo(3)–Mo(5) | 48.7(2) |
| O(38)#3–Mo(3)–Mo(5) | 133.8(2) | O(31)–Mo(3)–Mo(5) | 132.6(2) | O(15)–Mo(3)–Mo(5) | 89.74(19) |
| O(1)–Mo(4)–O(56) | 104.3(4) | O(1)–Mo(4)–O(59) | 101.9(4) | O(56)–Mo(4)–O(59) | 95.4(3) |
| O(1)–Mo(4)–O(25) | 95.6(4) | O(56)–Mo(4)–O(25) | 86.3(3) | O(59)–Mo(4)–O(25) | 161.3(3) |
| O(59)–Mo(4)–O(4) | 81.4(3) | O(25)–Mo(4)–O(4) | 80.2(3) | O(46)–Mo(4)–O(4) | 73.7(3) |
| O(1)–Mo(4)–Mo(10) | 100.9(3) | O(56)–Mo(4)–Mo(10) | 48.2(2) | O(59)–Mo(4)–Mo(10) | 48.4(2) |
| O(25)–Mo(4)–Mo(10) | 134.1(3) | O(46)–Mo(4)–Mo(10) | 134.3(2) | O(4)–Mo(4)–Mo(10) | 88.45(19) |
| O(6)–Mo(5)–O(57) | 107.1(4) | O(6)–Mo(5)–O(54) | 102.6(4) | O(57)–Mo(5)–O(54) | 95.3(3) |
| O(6)–Mo(5)–O(24) | 93.3(4) | O(57)–Mo(5)–O(24) | 87.5(3) | O(54)–Mo(5)–O(24) | 162.2(3) |
| O(6)–Mo(5)–Mo(3) | 101.1(3) | O(57)–Mo(5)–Mo(3) | 48.3(2) | O(54)–Mo(5)–Mo(3) | 49.0(2) |
| O(24)–Mo(5)–Mo(3) | 135.8(2) | O(37)#1–Mo(5)–Mo(3) | 134.5(2) | O(44)–Mo(5)–Mo(3) | 87.78(19) |
| O(41)–Mo(6)–O(39) | 105.6(4) | O(41)–Mo(6)–O(55) | 101.9(4) | O(39)–Mo(6)–O(55) | 94.8(3) |
| O(41)–Mo(6)–Mo(12) | 100.6(3) | O(39)–Mo(6)–Mo(12) | 47.8(2) | O(55)–Mo(6)–Mo(12) | 48.6(2) |
| O(45)#1–Mo(6)–Mo(12) | 133.1(2) | O(21)–Mo(6)–Mo(12) | 133.9(2) | O(8)#1–Mo(6)–Mo(12) | 87.6(2) |
| O(12)–Mo(7)–O(51) | 106.7(4) | O(12)–Mo(7)–O(34) | 101.8(4) | O(51)–Mo(7)–O(34) | 94.9(3) |
| O(12)–Mo(7)–O(53) | 97.3(4) | O(51)–Mo(7)–O(53) | 87.2(4) | O(34)–Mo(7)–O(53) | 159.3(3) |
| O(12)–Mo(7)–O(35) | 98.4(4) | O(51)–Mo(7)–O(35) | 154.2(3) | O(34)–Mo(7)–O(35) | 85.9(3) |
| O(53)–Mo(7)–Mo(11) | 134.9(3) | O(35)–Mo(7)–Mo(11) | 133.3(2) | O(29)#2–Mo(7)–Mo(11) | 87.51(18) |
| O(17)–Mo(8)–O(58) | 104.3(4) | O(17)–Mo(8)–O(48) | 102.1(4) | O(58)–Mo(8)–O(48) | 96.2(3) |
| O(17)–Mo(8)–O(36) | 93.8(4) | O(58)–Mo(8)–O(36) | 86.1(3) | O(48)–Mo(8)–O(36) | 162.8(3) |
| O(17)–Mo(8)–O(35) | 95.6(3) | O(58)–Mo(8)–O(35) | 158.9(3) | O(48)–Mo(8)–O(35) | 86.2(3) |
| O(36)–Mo(8)–Mo(2) | 134.3(2) | O(35)–Mo(8)–Mo(2) | 134.6(2) | O(29)#2–Mo(8)–Mo(2) | 90.08(19) |
| O(23)–Mo(9)–O(49) | 106.2(4) | O(23)–Mo(9)–O(50) | 102.3(4) | O(49)–Mo(9)–O(50) | 94.9(3) |
| O(50)–Mo(9)–O(15) | 80.4(3) | O(47)#3–Mo(9)–O(15) | 79.9(3) | O(31)–Mo(9)–O(15) | 72.1(3) |
| O(23)–Mo(9)–Mo(1) | 101.3(3) | O(49)–Mo(9)–Mo(1) | 47.8(2) | O(50)–Mo(9)–Mo(1) | 48.7(2) |
| O(47)#3–Mo(9)–Mo(1) | 133.5(2) | O(31)–Mo(9)–Mo(1) | 133.3(2) | O(15)–Mo(9)–Mo(1) | 88.0(2) |
| O(19)–Mo(10)–O(56) | 105.5(4) | O(19)–Mo(10)–O(59) | 101.8(4) | O(56)–Mo(10)–O(59) | 96.0(3) |
| O(19)–Mo(10)–Mo(4) | 101.7(3) | O(56)–Mo(10)–Mo(4) | 48.5(2) | O(59)–Mo(10)–Mo(4) | 48.8(2) |
| O(10)–Mo(11)–O(30) | 84.6(3) | O(9)–Mo(11)–O(33)#2 | 166.7(3) | O(51)–Mo(11)–O(33)#2 | 84.8(3) |
| O(34)–Mo(11)–O(33)#2 | 81.9(3) | O(10)–Mo(11)–O(33)#2 | 78.2(3) | O(30)–Mo(11)–O(33)#2 | 72.6(3) |
| O(9)–Mo(11)–Mo(7) | 102.1(3) | O(51)–Mo(11)–Mo(7) | 47.9(2) | O(34)–Mo(11)–Mo(7) | 49.0(2) |
| O(10)–Mo(11)–Mo(7) | 134.6(2) | O(30)–Mo(11)–Mo(7) | 134.0(2) | O(33)#2–Mo(11)–Mo(7) | 90.76(18) |
| O(20)–Mo(12)–O(39) | 105.8(4) | O(20)–Mo(12)–O(55) | 101.5(4) | O(39)–Mo(12)–O(55) | 95.3(3) |
| O(20)–Mo(12)–O(26)#1 | 94.3(4) | O(39)–Mo(12)–O(26)#1 | 86.6(3) | O(55)–Mo(12)–O(26)#1 | 162.8(3) |
| O(20)–Mo(12)–O(37) | 96.1(4) | O(39)–Mo(12)–O(37) | 156.7(3) | O(55)–Mo(12)–O(37) | 87.6(3) |
| O(26)#1–Mo(12)–Mo(6) | 134.6(2) | O(37)–Mo(12)–Mo(6) | 135.6(2) | O(44)#1–Mo(12)–Mo(6) | 89.8(2) |
| O(59)#2–Mn(1)–O(48)#2 | 85.5(3) | O(34)–Mn(1)–O(48) | 94.9(3) | O(34)#2–Mn(1)–O(48) | 85.1(3) |
| O(59)–Mn(1)–O(48) | 85.5(3) | O(59)#2–Mn(1)–O(48) | 94.5(3) | O(48)#2–Mn(1)–O(48) | 180.000(1) |
| O(50)–Mn(2)–O(54) | 96.2(3) | O(55)#1–Mn(2)–O(54)#1 | 82.2(3) | O(55)–Mn(2)–O(54)#1 | 97.8(3) |
| O(50)#1–Mn(2)–O(54)#1 | 96.2(3) | O(50)–Mn(2)–O(54)#1 | 83.8(3) | O(54)–Mn(2)–O(54)#1 | 180.0(2) |
| O(62)–Mn(3)–O(18) | 88.0(4) | O(62)–Mn(3)–O(11) | 88.7(4) | O(18)–Mn(3)–O(11) | 98.1(3) |
| O(62)–Mn(3)–O(5) | 103.2(4) | O(18)–Mn(3)–O(5) | 96.2(3) | O(11)–Mn(3)–O(5) | 161.8(4) |
| O(32)–P(1)–O(40) | 107.6(5) | O(3)–P(1)–O(45) | 109.2(5) | O(32)–P(1)–O(45) | 111.9(5) |
| O(40)–P(1)–O(45) | 106.8(5) | O(11)–P(2)–O(15) | 112.2(5) | O(11)–P(2)–O(44) | 109.9(5) |
| O(15)–P(2)–O(44) | 107.6(4) | O(11)–P(2)–O(8) | 111.1(5) | O(15)–P(2)–O(8) | 107.9(4) |
| O(53)–P(3)–O(36) | 110.0(5) | O(61)–P(4)–O(25) | 112.6(5) | O(61)–P(4)–O(43) | 112.4(6) |
| O(25)–P(4)–O(43) | 110.4(5) | O(61)–P(4)–O(27) | 112.6(5) | O(25)–P(4)–O(27) | 106.4(5) |
| O(47)–P(5)–O(38) | 107.3(5) | O(18)–P(6)–O(14) | 109.5(5) | O(18)–P(6)–O(24) | 110.9(5) |
| O(14)–P(6)–O(24) | 107.5(5) | O(18)–P(6)–O(26) | 112.5(5) | O(14)–P(6)–O(26) | 107.1(5) |
| O(10)–P(7)–O(13) | 108.6(5) | O(16)–P(8)–O(4) | 111.9(5) | O(16)–P(8)–O(29) | 110.9(4) |
| O(4)–P(8)–O(29) | 107.2(4) | O(16)–P(8)–O(33) | 111.4(5) | O(4)–P(8)–O(33) | 106.9(4) |
| P(2)–O(8)–Mo(1) | 126.1(4) | Mo(6)#1–O(8)–Mo(1) | 101.1(3) | P(7)–O(10)–Mo(11) | 137.3(5) |
| P(2)–O(11)–Mn(3) | 118.4(5) | P(2)–O(15)–Mo(3) | 125.4(4) | P(2)–O(15)–Mo(9) | 127.2(5) |
| Mo(3)–O(15)–Mo(9) | 100.1(3) | P(6)–O(18)–Mn(3) | 135.0(5) | Mo(6)–O(21)–Mo(1)#1 | 112.8(3) |
| P(6)–O(24)–Mo(5) | 132.0(5) | P(4)–O(25)–Mo(4) | 130.7(5) | P(6)–O(26)–Mo(12)#1 | 133.0(5) |
| P(8)–O(29)–Mo(8)#2 | 126.4(4) | P(8)–O(29)–Mo(7)#2 | 126.4(4) | Mo(8)#2–O(29)–Mo(7)#2 | 99.5(3) |
3 Results and discussion
3.1 The crystal structure of complex 1
The single-crystal X-ray analysis revealed that compound 1 consists of {Mo6P4} hexamers, manganese cations and lattice water molecules. As shown in Figs. 1 and 2, in each {Mo6P4}, there is a six-membered ring of oxo-bridged Mo atoms with alternating Mo–Mo single bonds (2.5870(2)–2.6053(2) Å), and there are four phosphate groups. The phosphorous atoms have P–O bond lengths in the range 1.492(1)–1.586(9) Å, while the O–P–O bond angles are in the range 105.9(5)°–112.6(5)°. As illustrated in Fig. 3, the Mn(1) and Mn(2) atoms are located at the octahedral MnO6 center, the Mn–O distances are ranging from 2.103(9) to 2.236(2)Å, and the O–Mn–O angles vary from 78.31(6)° to 180.0(1)°, while Mn(3) atoms adopt a five-coordinated geometry.

Molecular structure of [Mn2Mo12O30(HPO4)8(H2O)]12−.

Polyhedral representation of [Mn2Mo12O30(HPO4)8(H2O)]12−.
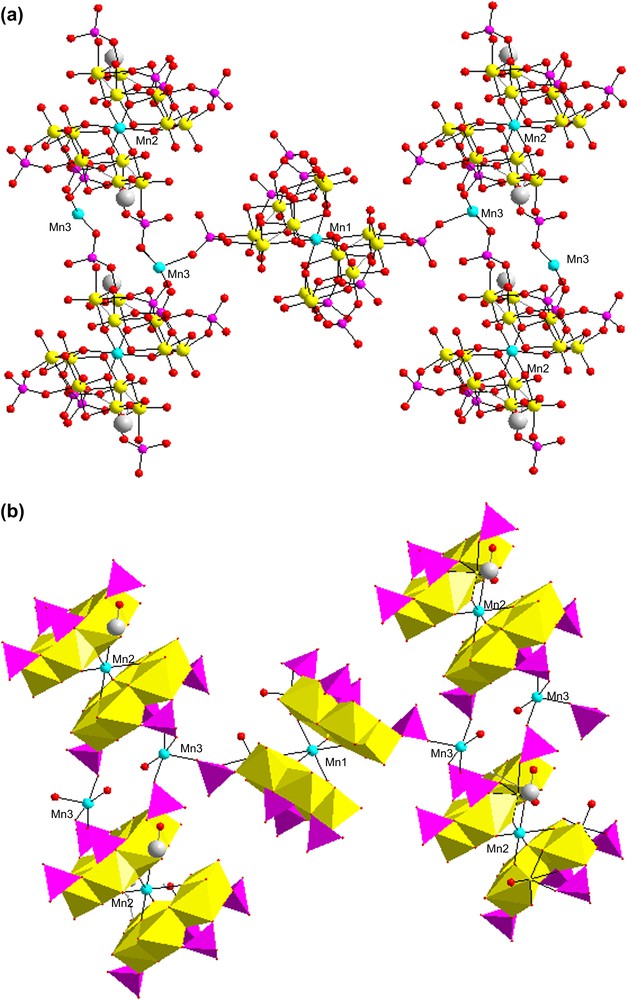
The two-dimensional framework of compound 1: (a) Ball and stick representation; (b) polyhedral view.
The structure of 1 was constructed from molybdenum hexamers bonded together with octahedral Mn2+ into a two-dimensional framework (see Fig. 3). There are three independent Mn atoms in the compound (see Fig. 3) which may be divided into two types: (i) Mn(1) and Mn(2), Mn(1) bridge two {Mo6P4} units via three μ3-O atoms, which link the metal–metal-bonded molybdenum atoms together with Mn–O bonds at lengths of 2.214(8) Å × 2, 2.217(8) Å × 2, and 2.192(7) Å × 2 to produce a centrosymmetric [Mn(Mo6P4)2] dimer. Mn(2) is similar to Mn(1). (ii) Mn(3) bridges four P–O groups from different {Mo6P4} (i.e. one {Mn(1)(Mo6P4)2} unit and two {Mn(2)(Mo6P4)2} units) and has a terminal coordinated water molecule O(1W) with the distance of Mn(3)–O(1W) 2.237(2) Å. Two-dimensional structure of 1 can be described in terms of crosslinking among different {Mn(Mo6P4)2} units. Interestingly,{Mn(1)(Mo6P4)2} unit and {Mn(2)(Mo6P4)2} unit are linked by P(7)O4 and Mn(3)O5, while {Mn(2)(Mo6P4)2} unit and {Mn(2)(Mo6P4)2} unit are linked by the oxygen atom of two P(5)O4, one P(2)O4, one P(6)O4 and two Mn(3)O5 polyhedra.
Bond-valence calculations for 1 suggest that the bond-valence values for all the molybdenum and phosphorous atoms are in the ranges 5.15–5.22 and 4.89–5.05, respectively. This indicates that all Mo and P atoms exhibit a +5 oxidation state. Each Mo5+ cation in compound 1 has a distorted octahedral configuration with an apical Mo–O bond at a length of 1.682(8)–1.694(9) Å and five other Mo–O bonds at lengths ranging from 1.932(8) to 2.343(7) Å. The valence sums for three Mn atoms are 1.93, 1.94 and 1.79, respectively, the average value being 1.89.
3.2 IR
The IR spectrum of compound 1 exhibits a strong band at 946 cm−1, characteristic of ν(Mo = O), and features at 727 and 1045 cm−1 associated with ν(Mo–O–M) and ν(P–O), respectively. The feature at 1517 cm−1 is ascribed to ethylenediamine, while the broad band at 3418 cm−1 is ascribed to water molecules [10].
4 Conclusion
A novel two-dimensional molybdenum(V) phosphate with manganese coordination cations has been hydrothermally synthesized and characterized by single-crystal X-ray diffraction. It may be concluded that the microstructures of molybdenum phosphates could be ‘tailored’ by structure-directing function of transition-metal coordination complex.
Supplementary material. The crystallographic data have been sent to the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk), with the deposited CCDC number 608620.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Fujian Province, China (No. E0510012) and Natural Science Foundation of JiaoYuTing, Fujian Province, China (No. JA04167).



Vous devez vous connecter pour continuer.
S'authentifier