1 Introduction
The modelling of the behaviour of hazardous materials under environmental conditions is among the most important applications of natural and technical sciences for the protection of the environment. In order to assess, for example, the safety of a geological repository for radioactive waste, it is essential to be able to predict the eventual dispersion of its hazardous components in the environment (geosphere and biosphere).
For hazardous materials stored in the subsurface or in geological formations, the most probable transport medium is the aqueous phase. An important factor is therefore the quantitative prediction of the reactions that are likely to occur between hazardous waste dissolved or suspended in groundwater, and the surrounding rock material, in order to estimate the quantities of waste that can be transported in the aqueous phase.
It is thus essential to know the relative stabilities of the compounds and complexes that may form under the relevant conditions. This information is often provided by speciation calculations using chemical thermodynamic data. Hence, equilibrium thermodynamics is one of the pillars which support safety analyses of repositories for radioactive waste. Thermodynamic constants are used in modelling reference pore waters, calculating radionuclide solubility limits, deriving case specific sorption coefficients, and last but not least in analysing experimental results. It is important to use the same database in all instances of this model chain to guarantee internally consistent results.
In the field of radioactive waste management, the hazardous material consists to a large extent of actinides and fission products from nuclear reactors, in addition to lesser amounts from other sources such as waste from medicine, industry and research facilities. The scientific literature on thermodynamic data, mainly on equilibrium constants and redox potentials in aqueous solution, has been contradictory in a number of cases, especially in actinide chemistry. A critical and comprehensive review of the available literature is necessary in order to establish a reliable thermochemical database that fulfils the requirements for rigorous modelling of the behaviour of the actinides and fission products in the environment.
The Radioactive Waste Management Committee (RWMC) of the Organisation for Economic Co-operation and Development (OECD) Nuclear Energy Agency (NEA) recognised the need for an internationally acknowledged, high-quality thermochemical database for application in the safety assessment of radioactive waste disposal, and initiated the development of the NEA Thermochemical Data Base (TDB) project in 1984 [1]. The RWMC assigned a high priority to the critical review of relevant chemical thermodynamic data of compounds and complexes for this area containing the actinides, including uranium, neptunium, plutonium and americium, as well as the fission product technetium. The first four books in the series on the chemical thermodynamics of uranium [2], americium [3], technetium [4], neptunium and plutonium [5] originated from this initiative.
In 1998, Phase II of the TDB Project (TDB-II) was started [1] to provide the further needs of the radioactive waste management programs by updating the existing database [6] and applying the TDB review methodology to the elements nickel [7], selenium [8] and zirconium [9], and to simple organic compounds and complexes, referred to here as the “NEA organics review” [10]. This paper presents a summary of the NEA organics review, with special emphasis on the results obtained for actinide compounds and complexes with the selected organic ligands. Detailed discussions and calculations, and all numerical values with their references (more than one thousand) are reported in Ref. [10].
2 Focus of the NEA organics review
A prerequisite for a successful and widely recognised critical evaluation of thermodynamic data is the sensible decision on which organic ligands the evaluation should comprise. This decision has two aspects, namely the importance of the ligands in radioactive waste problems, and the availability of experimental data. In the beginning of the project it was decided that the evaluation of organic ligands should be limited to oxalate, citrate, ethylenediaminetetraacetate (edta) and α-isosaccharinate (isa).
From the viewpoint of importance for radioactive waste problems, this set of ligands is very well posed.
Oxalate is, with respect to its complexation strength, the major product of radiolytic degradation of bitumen, sometimes used for waste conditioning [11], and of ion exchange resins used in decontamination procedures [12]. In addition, oxalate is one of the strong complexing organic ligands in nature (besides humic substances).
Citrate and edta are used in decontamination processes and thus, they become part of the radioactive waste inventory.
In terms of complexation strength, oxalate, citrate and edta cover a wide range of complex stability, and they may be used in model calculations as representatives of dicarboxylic acids (oxalate), hydroxy–polycarboxylic acids (citrate) and polyamino–polycarboxylic acids (edta).
Finally, from the viewpoint of complexation strength, isa is the most important product from the alkaline degradation of cellulose in cement pore waters [13]. Thus, isa is of major concern in many performance assessments of planned radioactive waste repositories.
Regarding the availability of experimental data, the situation is less clear. In the case of oxalate, citrate and edta, a large body of experimental studies has been published and the NEA organics review provides, based on the critical discussion of several hundreds of publications, a considerable set of selected thermodynamic values.
However, in the case of isa the number of experimental studies is very limited and, despite the importance of isa for performance assessments, only a few thermodynamic values could be selected. The critical review of experimental studies concerning isa is mainly a status report pointing out gaps in our present knowledge and further research needs.
It should be noted here that natural organic ligands, like humic and fulvic acids, have not been included in the NEA organics review. Although the interaction of humic and fulvic acids with actinides, especially in their trivalent oxidation state, can be of importance in radioactive waste problems, experimental data are scarce [27]. Moreover, the discussion concerning appropriate humic binding models is still ongoing [27–29], preventing a straightforward review procedure of thermodynamic data of natural organic ligands, in contrast to the simple organic ligands oxalate, citrate, edta and isa.
As the task of the NEA organics review is to complement the other reviews of the NEA TDB project [2–9], which are restricted to inorganic compounds and complexes of actinides and fission products, a natural choice of elements comprises U, Np, Pu, Am, Tc, Ni, Se and Zr.
However, the NEA organics review cannot be restricted to these elements as it aims at a thermodynamic data set useful for practical application. In addition to the above-mentioned actinides and fission products, the review considers also the major constituents of ground and surface waters which may interact with the selected organic ligands, i.e. H, Na, K, Mg and Ca. Any geochemical model including organic ligands should take into account these competing interactions and therefore, the NEA organics review provides a selected consistent set of these auxiliary constants.
3 Review procedure and results
The literature has been surveyed up to the end of 2001 for all ligands. For oxalate a few more recent references are included, and for isa the aim had been to consider all relevant literature up to 2004. The “golden age” of determination of chemical equilibrium constants in the 1970s is clearly depicted in Fig. 1. Nowadays, such work is mainly triggered by radioactive waste management needs.
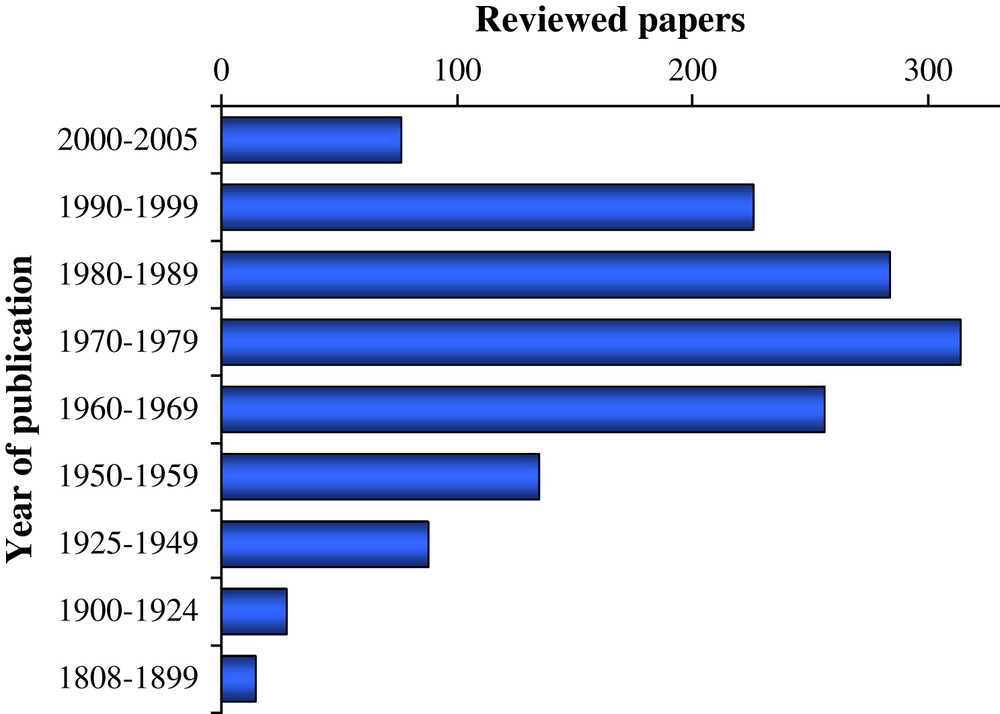
Scientific literature considered in the OECD/NEA TDB review on selected organic ligands.
Experimental measurements published in the scientific literature are the main source for the selection of recommended data. Previous reviews are not neglected. They have been primarily used as sources for original scientific literature, but they also form a valuable source of critical information on the quality of primary publications.
In the realm of metal–organic complexes, a plethora of experimental studies is found in the literature dealing with mixed complexes, i.e. complexes containing a common metal ion and two or more different ligands. In the NEA organics review mixed complexes, in general, were considered if they contain combinations of oxalate, citrate, edta and isa with or without additional inorganic ligands. Mixed complexes comprising other organic ligands are mentioned occasionally for qualitative comparison only.
From the viewpoint of application by far the most important class of mixed complexes are ternary metal–hydroxide–organic ligand complexes. These hydrolysed organic complexes may predominate in alkaline ground and surface waters and in high-pH cement pore waters and thus, they are important in assessing the influence of organic ligands on element complexation in cementitious repositories. The relevant literature about such complexes is discussed in the NEA organics review, but only in a few cases reliable thermodynamic constants could be selected.
Also of importance in many ground and surface waters would be the class of metal–carbonate–organic ligand complexes. However, the NEA organics review can only state the almost complete lack of such data.
When necessary, experimental source data were re-evaluated by using chemical models that are either found to be more realistic than those used by the original author, or are consistent with side-reactions discussed in another section of the review (for example, data on metal complex formation might need to be re-interpreted to take into account consistent values for ligand protonation reactions). Re-evaluation of literature values might also be necessary to correct for known systematic effects (for example, if metal–chloride complexation has been neglected in the original literature) or to make extrapolations to standard-state conditions (I = 0) by using the specific ion interaction theory (SIT). An example of such extrapolation to zero ionic strength is shown in Fig. 2.

Weighted least squares SIT-regression plot of equilibrium data measured in NaCl [14] for the formation of UO2edta2− according to the reaction UO22+ + edta4− ⇌ UO2edta2−. The data measured in KNO3 [15] are shown for comparison only.
4 Thermodynamic data of actinide compounds and complexes
In the following sections the results of the NEA organics review concerning actinide compounds (Table 1) and aqueous complexes (Table 2) are summarised in qualitative terms from the viewpoint of their application in environmental modelling studies. NEA selected thermodynamic data for reactions involving actinide compounds and complexes are summarised in Table 3. Their actual derivation from a large number of literature references is discussed in detail in Hummel et al. [10].
Summary of actinide solid compounds with selected organic ligands
| ox2− | cit3− | edta4− | isa− | |
| U3+ | – | – | – | – |
| Np3+ | – | |||
| Pu3+ | Pu2ox3·10H2O | |||
| Am3+ | ??? Am2ox3·nH2O | |||
| U4+ | U(ox)2·6H2O | Sol | Sol | |
| Np4+ | Np(ox)2·6H2O | Sol | ||
| Pu4+ | Pu(ox)2·6H2O | Sol | ||
| Am4+ | ||||
| UO2+ | – | |||
| NpO2+ | Sol | |||
| PuO2+ | Sol | – | ||
| AmO2+ | – | – | ||
| UO22+ | UO2ox·3H2O | Sol | ||
| NpO22+ | – | – | – | |
| PuO22+ | – PuO2ox·3H2O | – | – | |
| AmO22+ | – | – | – | |
| Ni2+ | Ni(ox)·2H2O | Sol | ||
| Ca2+ | Ca(ox)·H2O | Ca3(cit)2·4H2O | Sol | Ca(isa)2(cr) |
| H+ | H2ox·2H2O | H3cit·H2O | H4edta(cr) |
Summary of actinide aqueous complexes with selected organic ligands
| ox2− | cit3− | edta4− | isa− | |
| U3+ | – | – | – | – |
| Np3+ | – | ??? | Pu(III)–edta, Am(III)–edta | ??? |
| Pu3+ | Pu(ox)+, Pu(ox)2−, Pu(ox)33− | Am(III)–cit | Pu(edta)−, Pu(Hedta)(aq) | Am(III)/Eu(III)–isa |
| Am3+ | Am(ox)+, Am(ox)2−, Am(ox)33− | Am(cit)(aq), Am(cit)22−, Am(Hcit)+, Am(Hcit)2− | Am(edta)−, Am(Hedta)(aq), Am(edta)(OH)2− | Am(isa−3H)− |
| U4+ | Uox2+, U(ox)2(aq), U(ox)32−, U(ox)44−, ??? U–ox–OH | ??? | Uedta(aq), UedtaOH−, (UedtaOH)22−, Uedta(OH)22− | U(OH)4isa− |
| Np4+ | Np(ox)2+, Np(ox)2(aq), Np(ox)32−, ??? Np–ox–OH | ??? | Np(edta)(aq), U(IV)–edta–OH | Np(OH)3isa(aq), Np(OH)3(isa)2−, Np(OH)4isa−, Np(OH)4(isa)22− |
| Pu4+ | Pu(ox)2+, Pu(ox)2(aq), Pu(ox)32−, Pu(ox)44−, ??? OH | ??? | Pu(edta)(aq), Pu(edta)OH−, Pu(edta)(OH)22− | ??? Pu(isa−4H)−, Pu(isa−2H)22− |
| Am4+ | – | |||
| UO2+ | – | |||
| NpO2+ | NpO2ox−, NpO2(ox)23− | NpO2cit2− | NpO2edta3−, NpO2(Hedta)2−, NpO2(H2edta)−, NpO2edtaOH4− | ??? |
| PuO2+ | – PuO2ox−, PuO2(ox)23− | – | – PuO2edta3−, PuO2(Hedta)2− | |
| AmO2+ | – AmO2ox−, AmO2(ox)23− | – AmO2(Hedta)2− | ||
| UO22+ | UO2ox(aq), UO2(ox)22−, UO2(ox)34− | UO2cit−, (UO2)2(cit)22−, UO2(Hcit)(aq) | UO2edta2−, (UO2)2edta(aq), UO2(Hedta)− | UO2isa+, UO2(isa)2(aq), UO2(isa)3−, ??? UO22+– isa–OH |
| NpO22+ | – NpO2ox(aq), NpO2(ox)22− | – | – | |
| PuO22+ | – PuO2ox(aq), PuO2(ox)22− | – PuO2cit−, PuO2(cit)24− | – PuO2edta2−, PuO2(Hedta)−, PuO2(H2edta)(aq) | |
| AmO22+ | – | – | – | |
| Ni2+ | Ni(ox)(aq), Ni(ox)22− | Ni(cit)−, Ni(cit)24−, Ni(Hcit)(aq), Ni(H2cit)+ | Ni(edta)2−, Ni(Hedta)− | Ni(isa)+ |
| Ca2+ | Ca(ox)(aq), Ca(ox)22− | Ca(cit)−, Ca(Hcit)(aq), Ca(H2cit)+ | Ca(edta)2−, Ca(Hedta)− | Ca(isa)+, Ca(isa−H)(aq) |
| H+ | Hox−, H2ox(aq) | Hcit2−, H2cit−, H3cit(aq) | Hedta3−, H2edta2−, H3edta−, H4edta(aq), H5edta+, H6edta2+ | Hisa(aq) |
NEA selected thermodynamic data for reactions involving actinide compounds and complexes with selected organic ligands [10]
| Species | Reaction | log10 K° |
| UO2ox·3H2O(cr) | UO2ox(aq) + 3 H2O(l) ⇌ UO2ox·3H2O(cr) | 1.80 ± 0.27 |
| UO2ox(aq) | UO22+ + ox2− ⇌ UO2ox(aq) | 7.13 ± 0.16 |
| UO2(ox)22− | UO22+ + 2 ox2− ⇌ UO2(ox)22− | 11.65 ± 0.15 |
| UO2(ox)34− | UO22+ + 3 ox2− ⇌ UO2(ox)34− | 13.8 ± 1.5 |
| NpO2ox− | NpO2+ + ox2− ⇌ NpO2ox− | 3.9 ± 0.1 |
| NpO2(ox)23− | NpO2+ + 2 ox2− ⇌ NpO2(ox)23− | 5.8 ± 0.2 |
| Am(ox)+ | Am3+ + ox2− ⇌ Am(ox)+ | 6.51 ± 0.15 |
| Am(ox)2− | Am3+ + 2 ox2− ⇌ Am(ox)2− | 10.71 ± 0.20 |
| Am(ox)33− | Am3+ + 3 ox2− ⇌ Am(ox)33− | 13.0 ± 1.0 |
| UO2cit− | UO22+ + cit3− ⇌ UO2cit− | 8.96 ± 0.17 |
| (UO2)2(cit)22− | 2 UO22+ + 2 cit3− ⇌ (UO2)2(cit)22− | 21.3 ± 0.5 |
| UO2(Hcit)(aq) | UO22+ + Hcit2− ⇌ UO2(Hcit)(aq) | 5.0 ± 1.0 |
| NpO2cit2− | NpO2+ + cit3− ⇌ NpO2cit2− | 3.68 ± 0.05 |
| Am(cit)(aq) | Am3+ + cit3− ⇌ Am(cit)(aq) | 8.55 ± 0.20 |
| Am(cit)22− | Am3+ + 2 cit3− ⇌ Am(cit)22− | 13.9 ± 1.0 |
| Am(Hcit)+ | Am3+ + Hcit2− ⇌ Am(Hcit)+ | 6.5 ± 1.0 |
| Am(Hcit)2− | Am3+ + 2 Hcit2− ⇌ Am(Hcit)2− | 10.8 ± 1.0 |
| Uedta(aq) | U4+ + edta4− ⇌ Uedta(aq) | 29.5 ± 0.2 |
| UO2edta2− | UO22+ + edta4− ⇌ UO2edta2− | 13.7 ± 0.2 |
| (UO2)2edta(aq) | 2 UO22+ + edta4− ⇌ (UO2)2edta(aq) | 20.6 ± 0.4 |
| UO2(Hedta)− | UO22+ + Hedta3− ⇌ UO2(Hedta)− | 8.37 ± 0.10 |
| Np(edta)(aq) | Np4+ + edta4− ⇌ Np(edta)(aq) | 31.2 ± 0.6 |
| NpO2edta3− | NpO2+ + edta4− ⇌ NpO2edta3− | 9.23 ± 0.13 |
| NpO2(Hedta)2− | NpO2+ + Hedta3− ⇌ NpO2(Hedta)2− | 5.82 ± 0.11 |
| NpO2(H2edta)− | NpO2+ + H2edta2− ⇌ NpO2(H2edta)− | 4.47 ± 0.14 |
| Pu(edta)− | Pu3+ + edta4− ⇌ Pu(edta)− | 20.18 ± 0.37 |
| Pu(Hedta)(aq) | Pu3+ + Hedta3− ⇌ Pu(Hedta)(aq) | 1.84 ± 0.26 |
| Am(edta)− | Am3+ + edta4− ⇌ Am(edta)− | 19.67 ± 0.11 |
| Am(Hedta)(aq) | Am3+ + Hedta3− ⇌ Am(Hedta)(aq) | 2.17 ± 0.25 |
4.1 Actinide compounds with organic ligands
The detailed discussion of organic compounds has been restricted in the NEA organics review to the so-called “sparingly soluble” solids which may be of importance in environmental modelling studies. These are mainly metal oxalates, which were evaluated in some detail, whereas the generally rather soluble citrate, edta and isa compounds are discussed in qualitative terms (Table 1). In the field of actinide compounds with oxalate, the only selected value refers to U(VI)–oxalate, UO2ox·3H2O (Table 3). For the tetravalent actinides U(IV), Np(IV) and Pu(IV), thermodynamic data are reported for solids of the type An(ox)2·6H2O, but no data were selected in the NEA review. However, the available data can be used for scoping calculations and as guidelines for data estimation procedures. The same situation applies for Pu(III)–oxalate, Pu2ox3·10H2O, whereas the data for Am2ox3·nH2O are contradicting and inconclusive and represent the only gap in the database with respect to actinide compounds relevant for environmental modelling. From the viewpoint of model application in performance assessments, the most important solid is calcium oxalate, because the possible precipitation of this solid in many ground and surface waters can limit the concentration of dissolved oxalate to rather low levels.
4.2 Actinide aqueous complexes with organic ligands
4.2.1 Trivalent actinides
In aqueous media, americium exists as the trivalent ion except under strongly oxidising conditions, where the penta- and hexavalent trans-dioxo americyl cations, AmO2+ and AmO22+, are formed [3]. Thus, a sufficient number of experimental studies could be reviewed and thermodynamic data could be selected for Am(III) oxalate, citrate and edta complexes (Table 3).
A consistent picture emerges concerning the simple complexes. Whereas in the case of oxalate data have been selected for Am(ox)+, Am(ox)2− and Am(ox)33−, citrate may form Am(cit)(aq) and Am(cit)22− complexes, but edta forms only Am(edta)−, reflecting the increasing size and denticity of the ligands from bidentate (oxalate) to tridentate (citrate) to hexadentate (edta). Additional ligands may coordinate with Am(III), but the interactions are expected to be weak for steric reasons.
The data also reveal a consistent pattern for protonated complexes. The complexes Am(Hedta)(aq), Am(Hcit)+ and Am(Hcit)2− are important species in acidic solutions. Although complexes with Hox− may be possible, their stabilities are expected to be much smaller than those with ox2−. The few experimental data concerning protonated Am(III) oxalate complexes have been considered inconclusive in the organics review.
The selected values for Am(III) oxalate, citrate and edta complexes describe well the respective speciation in acidic and neutral to slightly alkaline solutions. However, in alkaline solutions mixed hydroxo complexes may also form.
In the case of edta, a single study proposed the complex Am(edta)(OH)2−. Although the data interpretation and the derived constant are not unreasonable, the organics review did not select a value based on a single study of uncertain accuracy. For citrate, mixed hydroxo complexes have also been proposed, but the data were considered as unreliable by the organics review. No information about mixed hydroxo complexes with oxalate could be found in the literature. However, one may infer from the analogous Am(III)–carbonate system, where no evidence for the formation of ternary hydroxide–carbonate complexes was found [6], that they are always minor species if they form at all.
For isa the complex Am(isa−3H)− has been proposed to form in alkaline solutions. The subscript −3H indicates the assumption that three protons dissociated from the alcoholic OH groups of isa− to form the Am(III)–isa complex in alkaline solutions. This stoichiometry is ambiguous, and the available experimental data may be more appropriately represented by the following reaction:
| Am(OH)3(aq) + isa− ⇌ Am(OH)3−x(isa−xH)− + xH2O | (1) |
No value has been selected for these complexes by the organics review. Nevertheless, the reported data are of value by providing “first estimates” of Am(III) isa complexation in alkaline solutions in the absence of more reliable data.
In the case of Pu(III), only equilibrium constants for the edta complexes Pu(edta)− and Pu(Hedta)(aq) could be selected in the organics review (Table 3). Their numerical values are consistent with the corresponding Am(III)–edta complexes. No data for the Pu(III)–edta system in alkaline solutions could be identified.
The data found for the Pu(III)–citrate system are not considered reliable enough by the organics review to derive selected values. However, the values reported for Pu(cit)(aq) and Pu(Hcit)+ may be used as guidelines, together with the selected Am(III)–citrate values, for scoping calculations and data estimation procedures.
Several Pu(III)–oxalate complexes have been proposed in the literature. Approximate values of equilibrium constants for Pu(ox)+, Pu(ox)2− and Pu(ox)33− are provided for qualitative modelling in the organics review, but no value could be selected.
No data for the Pu(III)–isa system could be found. The discussion of the Am(III)–isa and Eu(III)–isa systems in the organics review may serve as guidelines for qualitative modelling.
Only one experimental study of the Np(III)–edta system could be identified. However, the reported details about the measurements are too poor in order to base a selection on this single study. For environmental modelling studies, the data selected for Pu(III)–edta and Am(III)–edta might be considered as guidelines for data estimation procedures concerning Np(III)–edta complexes.
There is no evidence in the literature on the formation of Np(III)–oxalate complexes in aqueous solutions. However, Np(III) is oxidised by oxalate [16] and hence, the lack of data on unstable complexes is of no consequence for modelling of environmental systems.
No information about the Np(III)–citrate and the Np(III)–isa systems could be found in the literature. As it is not clear whether citrate and isa oxidise Np(III), as oxalate does, or may form stable complexes, as edta does, this lack of information may represent a real gap in the database.
No experimentally determined thermodynamic data could be identified in the organics review for U(III) oxalates, citrates, edta and isa. However, U(III) is unstable in aqueous solution and is thermodynamically capable of being oxidised to U(IV) by many anions [17]. For example, it was observed [17] that U(III) was almost instantaneously oxidised to U(IV) by ammonium oxalate, while oxalate was reduced to glyoxylic acid (OCHCOOH). Also, when edta is added to an U(III) acetate solution, the strongly complexing ligand edta does not stabilise the trivalent state of uranium, but accelerates its oxidation, which indicates that edta plays the role of an oxidising agent with respect to U(III) [17]. Hence, the lack of thermodynamic data for unstable U(III) complexes with organic ligands does not represent a gap in the database.
4.2.2 Tetravalent actinides
The tetravalent oxidation state of actinides is difficult to explore with respect to aqueous complexation. The very strong hydrolysis of U(IV), Np(IV) and Pu(IV) and the tendency of colloid formation in An(IV) aqueous solutions pose serious obstacles for experimental investigations.
Although colloid formation can be of importance in the environmental behaviour of An(IV) aqueous solutions, a general discussion of the colloidal properties of tetravalent actinides solutions was outside the scope of the NEA organics review. Colloid formation has been considered in the review of experimental data only with respect to its influence on the quality of thermodynamic data.
A few binary aqueous U(IV), Np(IV) and Pu(IV) oxalate complexes are reported in the literature (Table 2). Their stability constants are usually determined in conjunction with solubility measurements for solid An(IV) oxalates, which are associated with various shortcomings as discussed in detail in Ref. [10]. Hence, in spite of the qualitative self-consistency and the apparent reasonableness of the reported stability constants, no thermodynamic data for the complexes shown in Table 2 are recommended by the organics review. Nevertheless, the data may serve as qualitative guidelines in modelling exercises.
The mentioned solubility measurements of An(IV) oxalates have been carried out in acidic solutions in order to minimise problems with the very strong hydrolysis. A ternary complex, U(ox)2(OH)22−, was assumed to form when U(ox)2·6H2O was dissolved in solutions of ammonium bicarbonate, but no stability constant was determined. Generally, the behaviour of U(IV), Np(IV) and Pu(IV) oxalates in neutral and alkaline solutions is unexplored and the missing stability constants for ternary An(IV)–ox–OH complexes represent serious gaps in the database.
The situation is even worse in the case of U(IV), Np(IV) and Pu(IV) citrates. Either no data at all could be identified, as in the case of Np(IV) citrates, or the few published data for the U(IV) and Pu(IV) citrate systems are ambiguous with respect to the stoichiometry and stability of the formed complexes. Although the limited information for An(IV) citrates suggests very strong complex formation, the ambiguous data cannot be used for scoping calculations.
In the system U(IV)–edta, thermodynamic data for the complex Uedta(aq) could be selected by the organics review (Table 3). The hydrolysis of Uedta(aq) occurs already in acidic medium at pH > 3 with formation of UedtaOH− and of the dimeric species (UedtaOH)22−. In addition, the formation of Uedta(OH)22− has been reported at pH > 7. Although no values could be selected by the organics review for U(IV) edta hydrolysis species, they may serve as qualitative guidelines in modelling exercises.
Furthermore, thermodynamic data for the complex Np(edta)(aq) could be selected (Table 3), which is consistent with the Uedta(aq) data. The Np(IV)–edta–OH system has not been explored experimentally, but data for the analogous U(IV)–edta–OH mixed complexes can be used as qualitative guidelines.
In the case of Pu(IV)–edta, a paper of Boukhalfa et al. [18] became available to the reviewers only in the final stage of preparation of their review. The reported species and their stability constants are consistent with the U(IV) and Np(IV)–edta data and the experimental procedures seem to be reliable, but the results need to be re-evaluated using NEA auxiliary constants and the SIT approach before an eventual data selection can be made.
For the U(IV), Np(IV) and Pu(IV)–isa systems, the few reported studies qualitatively agree that isa forms very strong complexes with these tetravalent actinides in alkaline solutions. However, the proposed number and stoichiometry of formed complexes widely disagree (Table 2). The most detailed study has been published for the Np(IV)–isa system, but due to the uncertainties in oxidation state analysis, the scarcity of data and the uncertainty in defining the reaction stiochiometries, no thermodynamic values have been selected by the organics review. However, the results give an indication of the order of magnitude of Np(IV)–isa complexation and the reported values may be used for scoping calculations. They may also serve as guidelines for estimating U(IV) and Pu(IV)–isa complexation effects.
Tetravalent americium is unstable in non-complexing solutions and is reduced spontaneously to its more stable III oxidation state [19]. Am(IV) complexes can be obtained in the presence of high concentrations of strongly complexing agents such as carbonates [3]. A thermodynamic constant was selected for the complex AmIV(CO3)56− by the NEA americium review [3] based on experimental data of Bourges et al. [20]. In the latter study, Am(IV) was prepared electrochemically by anodic oxidation in sodium bicarbonate–carbonate medium 1.2 M < [HCO3− + CO32−] < 2.3 M in the pH range from 9.5 to 10. The Am(IV) solutions were “stable for ∼12 h in the best conditions” [20]. No study about the interaction of Am(IV) with oxalate, citrate, edta or isa could be found in the literature, except that Am(IV) is mentioned as reaction intermediate in the reduction of Am(VI) by oxalic acid [21] (see Section 4.2.4). However, this lack of experimental data probably is of no importance for environmental modelling studies, as we might infer from the Am(IV)–carbonate system that Am(IV) in the presence of oxalate, citrate, edta or isa will be slowly reduced to Am(III).
4.2.3 Pentavalent actinides
Oxalate complexes of U(V) were identified only as reaction intermediates in the reduction of U(VI). No experimental stability constants of aqueous U(V) oxalate complexes were identified in the literature. No information about U(V) citrate, edta or isa complexes could be found. There is no indication that U(V) could be stabilised by complexing ligands, and hence, the lack of thermodynamic data for unstable U(V) complexes with organic ligands does not represent a gap in the database.
Np(V) is the most common redox state of neptunium, reflected by a large body of experimental studies, which allowed us to select thermodynamic constants for Np(V) oxalate, citrate and edta complexes (Table 3).
In the Np(V)–oxalate system, values for the complexes NpO2ox− and NpO2(ox)23− have been selected. There is no evidence for the formation of higher complexes. A postulated protonated complex, NpO2Hox(aq), has been rejected by the organics review after re-analysis of a comprehensive experimental data set. On the other hand, although there were clear indications for the formation of mixed Np(V)–OH−–ox2− complexes at pH > 9, the sparse experimental data in the alkaline region do not allow to resolve ambiguities with respect to their stoichiometry.
A literature search by the organic review on the thermodynamics of neptunium–citrate systems revealed only information concerning the aqueous complexes of Np(V). Because of the small formal charge of Np(V) and the steric hindrance due to the linear dioxo structure O–Np–O, NpO2+ forms only one-to-one complexes with citrate. A value for the complex NpO2cit2− has been selected. Although the formation of a weak NpO2(Hcit)− complex is probable, the thermodynamic data reported are not reliable enough to be accepted. The formation of NpO2(cit)(OH)3− at pH > 9 has been claimed, the data are considered inconclusive by the organics review.
The complexation of Np(V) with edta has been studied extensively, and the organic review could select thermodynamic values for NpO2edta3−, NpO2(Hedta)2− and NpO2(H2edta)−. A single value reported for the species NpO2edtaOH4− has not yet been confirmed by any other study. Although the reported stability constant does not look unreasonable, indicating the formation of this species at pH > 11, the value is not selected by the organics review, but it may serve as qualitative guidelines in modelling exercises.
No information has been found for the Np(V)–isa system. As we may infer from the above discussion that Np(V)–isa complexes might be stable with respect to redox effects, and as there is no chemical analogue for this system, the lack of data represents a gap in the database.
Stability constants have been reported for Pu(V) oxalate and edta complexes (see Table 2). However, Pu(V) disproportionates strongly in oxalate solution [22] and forms Pu(IV) and Pu(VI) oxalate complexes. The Pu(VI) oxalate complex was observed to be slowly reduced to a Pu(IV) oxalate complex without the buildup of a Pu(V) oxalate complex [23]. Furthermore, Pu(VI) is reduced by edta to Pu(V) and finally to Pu(IV) [23]. Hence, no reliable stability constants are available for Pu(V) oxalate and edta complexes, and no values are selected in the organics review for these systems. No data could be found for the Pu(V) citrate and isa systems. However, there is no need to estimate values for highly unstable Pu(V) organic complexes, except perhaps for short-term laboratory studies involving the kinetics of Pu(V) disproportionation and reduction in the presence of organic ligands.
Am(V) in oxalate and edta solutions slowly changes into the trivalent state. The rate of reduction of 1 mM solution of Am(V) at 25 °C is similar in both cases, i.e. 2–3% per hour in 0.05 M edta [24], and about 2% in 0.1 M oxalate [25,26]. Stability constants were proposed in the literature for the complexes AmO2ox−, AmO2(ox)23−, and AmO2(Hedta)2−. Qualitatively, AmO2+ forms oxalate and edta complexes with similar stabilities as NpO2+ complexes, but no values were selected in the organics review because of various shortcomings in the experimental procedures. However, considering the instability of Am(V) in the presence of oxalate and edta, their values may only be of importance in short-term laboratory studies. They are of no importance in any long-term environmental modelling study.
No information about Am(V)–citrate and isa systems could be found, but we may infer that citrate and isa have similar reducing effects on Am(V) as oxalate and edta, as higher oxidation states of americium are generally reduced by organic complexing agents [19].
4.2.4 Hexavalent actinides
The U(VI)– oxalate, citrate and edta systems have been studied extensively, and in all cases the NEA organics review could select thermodynamic values for several complexes (Table 3). In the case of oxalate, only data for the mononuclear species UO2ox(aq), UO2(ox)22− and UO2(ox)34− were selected, whereas in the citrate and edta systems, data for dinuclear species also, (UO2)2(cit)22− and (UO2)2edta(aq), and complexes with protonated ligands, UO2(Hcit)(aq) and UO2(Hedta)−, were selected.
The existence of the protonated U(VI) oxalate complexes that were assumed to have formed in some experiments, UO2(Hox)+, UO2(Hox)2(aq) and UO2(H2ox)2+, is still open for debate. These species, difficult to identify by physical methods, were usually postulated to improve the fitting of potentiometric or spectrophotometric data. If they form, it is most likely that they occur in strongly acidic solutions. Taking into consideration the contradictory information in the literature on the presence of these complexes, stability constants reported in the literature were not accepted by the organics review.
A few dinuclear U(VI) oxalate complexes were assumed to exist in solutions, i.e. (UO2)2(ox)32− and (UO2)2(ox)56−. The inclusion of such species in models to interpret experimental data only slightly improved the overall fit, as they remained minor species (<5%) under the chosen experimental conditions. More studies are needed to confirm the presence of such complexes in solution and to obtain reliable stability constants.
A variety of ternary U(VI) oxalate complexes were assumed to exist in solution. However, thermodynamic data for such complexes are rare. Among the reported ternary complexes, the U(VI)–hydroxide–oxalate complexes are of some importance in predicting the chemical behaviour of U(VI) in pH neutral environments. Using the reported data on the U(VI)–hydroxide–oxalate complexes for scoping calculations, it is revealed that these ternary species could amount to 20–30% of the total U(VI) in the pH regions between 6 and 8. However, the stoichiometry of the formed complexes is ambiguous and further studies are needed to obtain reliable data on this system.
A similar situation is found in the U(VI)–citrate system: although the reported observations suggest the formation of U(VI) complexes with H−1cit4− (deprotonation of the alcoholic hydroxyl group of citrate) or of mixed hydroxide–citrate complexes in the pH regions between 6 and 8, the available data are insufficient and no conclusions can be drawn.
The linear O-U-O configuration of the uranyl ion allows edta to coordinate only in the equatorial plane. Hence, due to sterical hindrance the complex formally written as UO2edta2− (Fig. 2) might actually be the ternary complex UO2(Hedta)OH2−, forming above pH 5 from UO2(Hedta)− by the dissociation of a proton from a coordinated water molecule.
A variety of polynuclear U(VI)–edta species, including ternary U(VI)–hydroxide–edta complexes, have been suggested in the literature. No values for polymeric U(VI) edta other than (UO2)2edta(aq) are selected in the organics review, but as long as systems are modelled where edta is in excess with respect to uranium, these complexes are not of importance.
A complexation study of isa with U(VI) in acidic solutions could be interpreted in terms of the complexes UO2isa+, UO2(isa)2(aq) and UO2(isa)3−. Although their reported stability constants appear reasonable, more studies are needed to confirm them. The values may be used for scoping calculations, but they only describe the U(VI)–isa system in acidic solutions. In alkaline solutions, other complexes of the type UO2(OH)x(isa−yH)1−x−y may dominate the U(VI)–isa system.
The reduction of Np(VI) and Pu(VI) by oxalate, citrate and edta has been investigated in low-ionic-strength media and brines [23]. At low ionic strength, Np(VI) was rapidly reduced to form Np(V) organic complexes, whereas Pu(VI) was predominantly reduced to Pu(IV). The presence of organic complexants also led to the rapid reduction of Np(VI) and Pu(VI) in brines, except in cases where carbonate and hydrolytic complexes predominated.
Hence, it is no surprise that no thermodynamic data have been found in the organic review for Np(VI) citrate, edta and isa complexes, and solely approximate values for Np(VI) oxalate complex formation are reported. However, these values are only of qualitative significance for laboratory studies. For any application modelling the long-term behaviour of neptunium, e.g., for nuclear waste disposal, the unstable Np(VI) oxalate complexes and the lack of experimental data for the Np(VI) citrate, edta and isa systems are of no importance, and there is no need to estimate values for highly unstable Np(VI) organic complexes.
Pu(VI) complexes with oxalate, citrate and edta have been proposed in the literature (see Table 2). However, none of these proposals has been considered reliable and no thermodynamic values are selected in the organics review. Considering the instability of Pu(VI) organic solutions, this non-selection has no consequences for environmental modelling studies.
Powerful oxidants oxidise Am(III) and Am(V) to Am(VI), which forms the linear trans-dioxo americyl cation AmO22+ [19]. In solutions with excess oxalic acid, Am(VI) was found to be rapidly reduced by oxalate to Am(IV), which disproportionates into Am(III) and Am(V) [21]. Also, the simultaneous formation of Am(V) and Am(III) was observed in solutions of edta, citrate and tartrate, although not all the reactions proceeded rapidly, and the yield of Am(III) depended on the pH [21]. No information is available for Am(VI)–isa interaction, but we may infer that isa has the same reducing effect on Am(VI) as citrate and tartrate. No thermodynamic data are available for these highly unstable Am(VI) complexes.
5 Summary and conclusions
A detailed literature review of thermodynamic data of compounds and complexes of U, Np, Pu and Am with the selected organic ligands oxalate, citrate, edta and isa has been carried out within the scope of the OECD/NEA Thermodynamic Data Base (TDB) project.
Thermodynamic data of solid compounds could be selected for Ca oxalate, citrate and isa, as well as for U(VI) oxalate. However, although the matrix of possible actinide–organic ligand compounds is almost empty (Table 1), no serious gaps have to be reported from the viewpoint of environmental modelling studies. The compounds formed by citrate, edta and isa are generally highly soluble salts. Some of the actinide–oxalate compounds have low solubilities, but in environmental studies the concentration of oxalate in solution can be assumed to be limited by Ca oxalate precipitation, and the formation of actinide–oxalate compounds is unlikely as long as the dissolved actinides do not exceed trace-level concentrations.
As a result of the NEA TDB organics review, a number of reliable values could be selected for aqueous complexes of oxalate, citrate and edta with actinides in their most common redox states, i.e. with Am(III), Np(V) and U(VI) (Table 3), as well as with the fission product Ni and the major competing elements Ca and H. In the cases of Pu(III), U(IV) and Np(IV), only data for edta complexes could be selected (Table 3). For isa, selected values could only be derived for Ca and H (Table 2).
The organic ligands considered in the NEA TDB organics review not only form strong complexes with actinides, they also influence the redox state of the actinides in aqueous solutions. Hence, two kinds of gaps in the matrix of possible actinide–organic ligand complexes (Table 2) were encountered.
- (1) Missing or unreliable thermodynamic data, because the actinide in solution is oxidised or reduced in the presence of the organic ligand and thus, the actinide–organic complexes formed in that unstable redox state are short-lived species only. These unstable redox states in the presence of organics are U(III), U(V), Pu(V), Am(V), Np(VI), Pu(VI) and Am(VI). These gaps in the database are of no importance from the viewpoint of environmental modelling studies or performance assessments for radioactive waste disposal.
- (2) Missing or unreliable data in stable and important redox states. These gaps mainly concern aqueous complexes of oxalate, citrate and isa with the tetravalent actinides U(IV), Np(IV) and Pu(IV). Furthermore, the systems Np(V)– isa in general and U(VI)–isa in alkaline solutions are terra incognita so far. From the viewpoint of performance assessment, studies these are the real gaps which need to be filled either by case specific estimates or by additional experimental studies.
Acknowledgements
The financial support of the organisations participating in NEA TDB Phase II is greatly acknowledged. The organics review received extensive help and encouragement from the NEA secretariat. Thanks to M. Illemassène for providing the French abstract.


