1 Introduction
Chrysanthemum trifurcatum (Desf.) Batt. and Trab. var. macrocephalum (viv.) Beg. (Asteraceae), spreading in Tunisia, is a herb with small yellow flowers. In Tunisian traditional medicine, the flowerheads of C. trifurcatum were used to treat constipation, intestinal transit problems and to fight feminine pain after delivery [1]; no other utilizations of this plant were reported previously [2].
Aromatic and medicinal plants produced a wide variety of volatile terpene hydrocarbons and their corresponding oxygenated derivatives were known as essential oils. In fact, essential oils have been widely used in traditional medicine. Among others, antibacterial, antifungal, immunomodulatory, antiinflammatory, and antirheumatic activities have been described [3–5]. Hitherto, there is only few information on the effects of essential oils on viruses or viral infections. Recently, the anti-herpes activity of several essential oils of different plant sources as well as of various constituents of essential oils was demonstrated [6–9].
A series of studies has demonstrated the potential medicinal effect of essential oils from various Chrysanthemum species, i.e. essential oils from air-dried and processed flowers of C. indicum possessed significant antimicrobial activity effect [10], essential oil of C. boreale exhibited antibacterial activity against Gram positive and Gram negative bacteria [11], volatile fraction of C. viscidehirtum aerial parts exhibited activity against some bacterial strains, in particular Salmonella typhi and Proteus mirabilis [12], essential oil of flowerheads of C. coronarium possessed antifungal activity against agricultural pathogens [13]. Concerning the antiviral activity of Chrysanthemum species, only C. cinerariaefolium was reported to have an antiviral effect against the Herpes simplex virus which has been attributed to pyrethrins [14].
To the best of our knowledge, the chemical composition of essential oil of C. trifurcatum has not been studied yet, as well as its antimicrobial properties. The aim of this work was to investigate the chemical composition, antibacterial and antiviral activities of essential oil from C. trifurcatum flowerheads growing in Tunisia.
2 Material and methods
2.1 Plant material
C. trifurcatum was identified according to the flora of Tunisia [15] by the botanist Dr. Fethia Harzallah-Skhiri (Institut supérieur de biotechnologie de Monastir, Tunisia). Fresh flowerheads of C. trifurcatum were collected from Zeramdine in the center of Tunisia in May 2006. A voucher specimen was deposited in our laboratory for future reference.
2.2 Essential oil extraction and analysis
2.2.1 Essential oil extraction
Fresh flowerheads of C. trifurcatum were submitted to hydrodistillation for 5 h using a Clevenger type apparatus. The obtained essential oil was dried with anhydrous sodium sulphate and stored at 4 °C before use. The yield based on fresh weight of the sample was calculated.
2.2.2 Essential oil analysis
The composition of the oil was analysed by GC and GC/MS. The analytical GC was carried out on an HP5890-series II gas chromatograph (Agilent Technology, California, USA) equipped with Flame Ionization Detectors (FID) under the following conditions: the fused silica capillary column, apolar HP-5 and polar HP Innowax (30 m × 0.25 mm ID, film thickness 0.25 μm). The oven temperature was held at 50 °C for 1 min, then programmed at rate of 5 to 240 °C/min and held isothermal for 4 min. The carrier gas was nitrogen at a flow rate of 1.2 ml/min; injector temperature: 250 °C, detector temperature: 280 °C; the volume injected: 0.1 μl of 1% solution (diluted in hexane). The percentages of the constituents were calculated by electronic integration of FID peak areas without the use of response factor correction.
GC/MS was performed in a Hewlett-Packard 5972 MSD system. An HP-5 MS capillary column (30 m × 0.25 mm ID, film thickness of 0.25 μm) was directly coupled to the mass spectrometry. The carrier gas was helium, with a flow rate of 1.2 ml/min. Oven temperature was programmed (50 °C for 1 min, then 50–240 °C at 5 °C/min) and subsequently held isothermal for 4 min. Injector port: 250 °C, detector port: 280 °C, split ratio 1:50. Volume injected: 0.1 μl of 1% solution (diluted in hexane). Mass spectrometer: HP5972 recording at 70 eV; scan time 1.5 s; mass range 40–300 amu. Software adopted to handle mass spectra and chromatograms was a ChemStation. The components were identified by comparison of their mass spectra with those in the Wiley 275 GC–MS library and those in the literature [16], as well as by comparison of their retention indices with literature data [16–20]. Retention indices of the components were determined relative to the retention times of a series of n-alkanes (relative to C9–C28 on the HP5 and HP-20M columns).
2.3 Antibacterial activity
2.3.1 Bacterial strains
Bacterial strains were obtained from the collections of the Pasteur Institute in Paris, France. Microorganisms were as follows: five Gram positive bacteria (Staphylococcus epidermidis CIP 53124, Staphylococcus hoemolyticus CIP 8156, Staphylococcus hominis CIP 8157, Staphylococcus simulans CIP 8164 and Bacillus subtilis CIP 5265) and three Gram negative bacteria (Escherichia coli CIP 54117, Hafnia alvei CIP 5731 and clinical strain P. mirabilis). Organisms were maintained in nutrient agar (Sigma) at 37 °C. Overnight cultures were prepared in Mueller Hinton Broth (Sigma) and adjusted to approximately 108 cfu/ml.
2.3.2 Microdilution method
Antibacterial evaluation of the essential oil was performed in 96-well plates. Samples of cultures grown overnight (108 cfu/ml) were incubated with oil dissolved in ethanol 99% (at a concentration of 1, 10, 100 and 500 μg/ml) for 24 h at 37 °C. All inhibition assays were carried out in triplicate. Growth was monitored by measuring OD600 with a Packard Spectracount microplate spectrophotometer and the percentage of inhibition (%I) was calculated for each concentration [21]:
| %I = (ODc − ODt)/ODc × 100 |
2.4 Determination of cytotoxicity by cell viability
2.4.1 Cell strain
African green monkey kidney cells (Vero, ATCC CCL-81) were grown in Eagle's Minimum Essential Medium (MEM, Eurobio), supplemented with 8% Fetal Calf Serum (FCS, Eurobio) and 1% of antibiotics PCS (10 000 IU Penicillin/ml, 25 000 IU Colimycin/ml, 10 mg Streptomycin/ml; Sigma).
2.4.2 Cytotoxicity assay
To evaluate the cytotoxic activity of the oil, Vero cellular suspensions (3.5 × 105 cells/ml) were cultivated in 96-well culture plates and exposed to increasing concentrations of the oil from 10 to 1000 μg/ml, using 4 wells for each concentration. The plates were incubated at 37 °C in a humidified CO2 atmosphere (5% CO2) during 72 h. Each assay was done in triplicate. The cells were examined daily under a phase-contrast microscope to determine the minimum concentration of oil that induced alterations in cell morphology, including swelling, shrinkage, granularity and detachment [22]. Cytotoxicity was tested using the neutral red dye method [23], and optical densities (OD) were measured at 540 nm using a spectrophotometer (SpectraCount™, Packard). The 50% cytotoxic concentration (CC50) was the concentration of the oil that inhibited actively the replication of cells by 50% of untreated ones. Cytotoxicity was also expressed as the percentage of cell destruction (%D):
| %D = [((ODc)C − (ODc)Mock)/(ODc)C] × 100. |
(ODc)C and (ODc)Mock were the OD values of the untreated and treated cells, respectively [24].
2.5 Antiviral assays by cell viability
2.5.1 Viral strain
HSV-1 (wild type strain 17, sensitive to acyclovir) was obtained from Pr of Ingrand (Hôpital Antoine-Béclère, Rheims, France). The virus titer was estimated from cytopatogenicity according to the Reed and Muench dilution method [25], and expressed as 50% infectious doses per milliliter (ID50/ml). The HSV-1 stock had a titer of 2 × 105.8 ID50/ml. The used viral suspension has a multiplicity of infection (MOI) of 0.001 ID50/ml.
2.5.2 Antiviral assay
To test the antiviral effect of oil, 100 μl of Vero cellular suspension (3.5 × 105 cells/ml) were infected with a virus (HSV-1) suspension (50 μl) with a multiplicity of infection (MOI) of 0.001 ID50/ml without or in presence of different dilutions of the oil (10–1000 μg/ml). Infected cell cultures were cultivated in 96-well culture plates at 37 °C in a humidified CO2 atmosphere (5% CO2) during 72 h. Each assay was done in triplicate. After incubation, antiviral activity was evaluated by the neutral red dye method [23]. The antiherpetic compound acyclovir [9-(2-hydroxyethoxymethyl) guanine] was used as reference drug with concentrations ranging from 0.1 to 5 μg/ml. The 50% effective antiviral oil concentration (EC50) was expressed as the concentration of the oil that achieved a protection of 50% of virus infected cells. Optical densities (OD) were measured at 540 nm and the OD was related directly to the percentage of viable cells, which was inversely related to the cytopathic effect (CPE). The linear regression was determined for each assay on the basis of cell controls (0% CPE) and virus controls (100% CPE). Data were expressed as a percentage of cell protection (%P):
| %P = [((ODt)virus − (ODc)virus)/((ODc)Mock − (ODc)virus)] × 100. |
(ODt)virus was the OD of the virus infected cell suspensions in the presence of oil; (ODc)virus was the OD of the virus infected cell suspensions (no oil) and (ODc)Mock was the OD of the mock-infected cell suspensions [24].
2.6 Statistical analysis
The percentage of bacterial growth inhibition was calculated for each bacterial strain as described previously and results were expressed as the mean of three replicates. The 50% inhibitive concentrations (IC50) were estimated by regression analysis with Prism software (GraphPad Software, Inc). The results of cytotoxicity and antiviral activity (%D and %P) were expressed as mean ± s.e.m.
3 Results and discussion
3.1 Chemical composition of the essential oil
Essential oil obtained by hydodistillation of fresh flowerheads of C. trifurcatum had a light yellow colour and a pungent odour at room temperature. The yield oil was 0.055% ((v/w), volume/fresh weight). Qualitative and quantitative analytical results by GC and GC/MS are shown in Table 1.
Composition of essential oil of C. trifurcatum var. macrocephalum flowerheads
| Compound | RIa | % | Identification |
| 2-Hexenal | 860 | 4.85 | MS, RI |
| Tricyclene | 930 | 0.27 | MS, RI |
| α-Thujene | 935 | 1.23 | MS, RI |
| α-Pinene | 940 | 5.32 | MS, RI |
| Camphene | 952 | 0.15 | MS, RI |
| Sabinene | 976 | 0.34 | MS, RI |
| β-Pinene | 979 | 8.77 | MS, RI |
| β-Myrcene | 991 | 2.31 | MS, RI |
| Limonene | 1032 | 20.89 | MS, RI |
| 1,8-Cineole | 1035 | 10.64 | MS, RI |
| γ-Terpinene | 1064 | 19.13 | MS, RI |
| α-Terpinolene | 1089 | 0.31 | MS, RI |
| 1-Octen-3yl-acetate | 1096 | 0.25 | MS, RI |
| Camphor | 1145 | 0.91 | MS, RI |
| Borneol | 1169 | 0.59 | MS, RI |
| Terpinen-4-ol | 1180 | 0.34 | MS, RI |
| p-Cymen-8-ol | 1187 | 0.28 | MS, RI |
| α-Terpineol | 1191 | 0.29 | MS, RI |
| Myrtenal | 1193 | 0.78 | MS, RI |
| Myrtenol | 1196 | 0.47 | MS, RI |
| Fenchyl acetate | 1225 | 0.13 | MS, RI |
| Carveol | 1229 | 0.27 | MS, RI |
| Linalyl acetate | 1262 | 0.17 | MS, RI |
| Bornyl acetate | 1289 | 0.26 | MS, RI |
| Carvacrol | 1291 | 0.40 | MS, RI |
| 4-Terpenyl acetate | 1340 | 3.42 | MS, RI |
| α-Cubebene | 1352 | 0.21 | MS, RI |
| α-Terpenyl acetate | 1354 | 0.17 | MS, RI |
| α-Ylangene | 1372 | 0.16 | MS, RI |
| α-Copaene | 1379 | 0.16 | MS, RI |
| β-Bourbobene | 1381 | 1.06 | MS, RI |
| β-Elemene | 1390 | 0.60 | MS, RI |
| β-Cubebene | 1401 | 0.13 | MS, RI |
| Longifolene | 1403 | 1.39 | MS, RI |
| α-Gurjunene | 1409 | 0.17 | MS, RI |
| β-Caryophyllene | 1420 | 0.31 | MS, RI |
| β-Gurjunene | 1434 | 0.31 | MS, RI |
| α-Cedrene | 1437 | 0.32 | MS, RI |
| α-Himachalene | 1447 | 0.22 | MS, RI |
| β-Farnesene | 1458 | 0.16 | MS, RI |
| Germacrene-D | 1484 | 0.23 | MS, RI |
| β-Selinene | 1489 | 0.20 | MS, RI |
| α-Muurolene | 1502 | 0.17 | MS, RI |
| β-Bisabolene | 1508 | 0.12 | MS, RI |
| γ-Cadinene | 1516 | 0.26 | MS, RI |
| δ-Cadinene | 1523 | 0.13 | MS, RI |
| α-Cadinene | 1538 | 0.13 | MS, RI |
| α-Calacorene | 1545 | 0.55 | MS, RI |
| Elemol | 1549 | 0.32 | MS, RI |
| Germacrene-B | 1555 | 2.01 | MS, RI |
| β-Calacorene | 1561 | 0.43 | MS, RI |
| β-Spathulenol | 1576 | 1.62 | MS, RI |
| τ-Cadinol | 1640 | 0.86 | MS, RI |
| τ-Muurolol | 1642 | 0.24 | MS, RI |
| β-Eudesmol | 1649 | 0.68 | MS, RI |
| α-Cadinol | 1654 | 1.39 | MS, RI |
| Monoterpene hydrocarbons | 58.72 | ||
| Oxygenated monoterpenes | 18.65 | ||
| Sesquiterpene hydrocarbons | 9.45 | ||
| Oxygenated sesquiterpenes | 5.34 | ||
| Aldehyde | 5.63 | ||
| Total identified | 97.48 |
a RI: retention indices relative to n-alkanes on the apolar HP-5 column.
The global chromatographic analysis of this essential oil showed 56 compounds, representing 97.48% of the total oil constituents (Table 1). The oil contains a complex mixture consisting of mainly mono- and sesquiterpene hydrocarbons and oxygenated mono- and sesquiterpenes. It was dominated by monoterpene hydrocarbons (58.72%) and oxygenated monoterpenes (18.65%), while sesquiterpene hydrocarbons and oxygenated sesquiterpenes were only present in small percentage with, respectively, 9.45 and 5.34%. The aldehyde components accounted for 5.63%. The major components in the oil detected were limonene (20.89%), γ-terpinene (19.13%), 1,8-cineole (10.64%), β-pinene (8.77%), α-pinene (5.32%), 2-hexenal (4.85%), 4-terpenyl acetate (3.42%), β-myrcene (2.31%), germacrene-B (2.01%), β-spathulenol (1.62%), longifolene (1.39%), α-cadinol (1.39%), α-thujene (1.23%) and β-bourbobene (1.06%) (Table 1).
To the best of our knowledge, this is the first report of the chemical composition of the essential oil of C. trifurcatum flowerheads. Neverthless, some other species in genus Chrysanthemum were studied for their essential oil compositions. Limonene, the major component of the oil, was in the same way present in higher quantity in the volatile fraction of C. viscidehirtum aerial parts [12]. On the contrary, limonene was present in minor quantity (0.7%) in the essential oil of C. coronarium flowerheads [13] which contain mainly camphor (29.2%), α-pinene (14.8%), lynalyl acetate (9.8%), β-pinene (9.5%) and camphene (5.2%) [13]. Moreover, 1,8-cineole was a major component in the essential oil of C. indicum flowerheads with 30.41% [10], and in the volatile fraction of C. boreale [11]. We can affirm that C. trifurcatum from Tunisia (Zeramdine) was the chemotype limonene, γ-terpinene and 1,8-cineole.
3.2 Antibacterial activity
The essential oil was evaluated for antibacterial activity against pathogenic strains of Gram positive (S. epidermidis, S. hoemolyticus, S. hominis, S. simulans and B. subtilis) and Gram negative (E. coli, H. alvei and P. mirabilis) bacteria. The mean percentage of bacterial growth inhibition and IC50 are given in Table 2. Growth inhibition percentages have been calculated for all concentration ranges of the tested oil and we reported here the data (%I) corresponding to the concentration 500 μg/ml of oil which shows a bacterial growth inhibition. The oil was active against the tested bacterial strains. However, this activity varies with the kind of bacteria. In fact, at the concentration of 500 μg/ml; essential oil exhibited potent inhibitory effect on the growth of two Gram positive strains: S. epidermidis (66%) and B. subtilis (64%) with IC50, respectively, for 62.5 and 125 μg/ml; this activity was more pronounced than the standard antibiotic (ampicillin) against those two bacteria. Moreover, this oil was inactive against the other tested microorganisms, with IC50 superior to 500 μg/ml. Our results indicated that Gram positive bacteria were more sensitive to the essential oil than Gram negative ones. This is in agreement with observations made by other authors that Gram positive bacteria were more susceptible to essential oils than Gram negative ones [26,27].
Antibacterial activities (%I and IC50 in μg/ml) of the essential oil of C. trifurcatum flowerheads
| Microorganisms | Essential oil | Ampicillin | ||
| %I | IC50 | %I | IC50 | |
| Staphylococcus epidermidis CIP 53124 | 66 | 62.5 | 20 | >500 |
| Staphylococcus simulans CIP 8164 | 20 | >500 | 32 | >500 |
| Staphylococcus hominis CIP 8157 | 47 | >500 | 32 | >500 |
| Staphylococcus haemolyticus CIP 8156 | 23 | >500 | 85 | 1.25 |
| Bacillus subtilis CIP 5265 | 64 | 125 | 30 | >500 |
| Escherichia coli CIP 54117 | 33 | >500 | 92 | 25 |
| Hafnia alvei CIP 5731 | 20 | >500 | 82 | 1.25 |
| Proteus mirabilis | 27 | >500 | 85 | 1.25 |
Our results were in accord with reported ones. In fact, essential oils of many Chrysanthemum species were known to exhibit antimicrobial activity against several bacteria and fungi [10,11,12,28]. The antibacterial activity of the oil could, in part, be associated with major constituents such as limonene, γ-terpinene 1,8-cineole, α-pinene, β-pinene and 2-hexenal. Limonene, the most abundant constituent (20.89%) of the oil, was known to exhibit antibacterial activity [29]. One of the major components of this oil, 1,8-cineole, has been previously shown to be active against many organisms [30,31] and it has been known to exhibit antibacterial activity against Bacillus subtillis, Staphylococcus aureus, E. coli and other bacteria [32]. α-Pinene and β-pinene, which were found to be in appreciable amounts in the oil of C. trifurcatum flowerheads, have been reported to display antibacterial effects [29,33]. Terpenes were active against bacteria [34]. In our case, the presence of high quantities of monoterpenoid components in essential oil could explain its antibacterial activity against Gram positive bacteria. Research on the antimicrobial actions of monoterpenes suggests that they diffuse into and damage cell membrane structures [35]. In fact it seems, as described previously by Juliani et al. [36], that essential oils containing terpenoids are more active against Gram positive organisms than against Gram negative ones. In addition, the components in lower amount may also contribute to antibacterial activity of the oil, involving probably some type of synergism with the other active compounds.
3.3 Cytotoxicity and antiviral activity
Essential oil of C. trifurcatum flowerheads was investigated against HSV-1 infected Vero cells. Results are summarized in Fig. 1. Assessment of cytotoxicity is clearly an important part of the evaluation of a potential antiviral agent because a useful oil should be selective for virus specific processes with no or only few effects on cellular metabolism. In Vero cells and after 72 h exposure, cytotoxicity of the oil varies within concentrations. In fact, at higher tested doses, the oil exhibited a potent percentage of cell destruction. At a concentration of 1000 μg/ml, oil destructed cells by 63% (Fig. 1). At lower doses, the oil was tolerated by Vero cells and its 50% cytotoxic concentration (CC50) was 735.9 μg/ml. In literature, many essential oil components were known to exhibit cytotoxic activity. β-elemene has been reported to have stronger cytotoxicity against tumor cells [37,38]. α-Cadinol has been reported to have toxicity against human colon adenocarcinoma cell line HT-29 [39], whereas both these sesquiterpene compounds were present in small percentage in the flowerheads' oil of C. trifurcatum. Concerning the cytotoxic potential of Chrysanthemum species, Ukiya et al. [40] reported that arnidiol, a triterpene isolated from edible Chrysanthemum flowers, exhibited remarkable cytotoxic activity against a panel of human cancer cell lines, and authors suggested that this compound can be useful as an anticancer agent. The essential oil of C. trifurcatum flowerheads did not exhibit a significant antiviral activity against H. simplex virus type 1. The percentage of virus-infected cells protection was lower (4.1% at a dose of 100 μg/ml) (Fig. 1). The reference standard, acyclovir (1 μg/ml), conferred total protection (100%, EC50 = 0.35 μg/ml) against HSV-1 with a low percentage of cell destruction. In the literature, only C. cinerariaefolium has an antiviral activity against H. simplex virus, which has been attributed to pyrethrins [14].
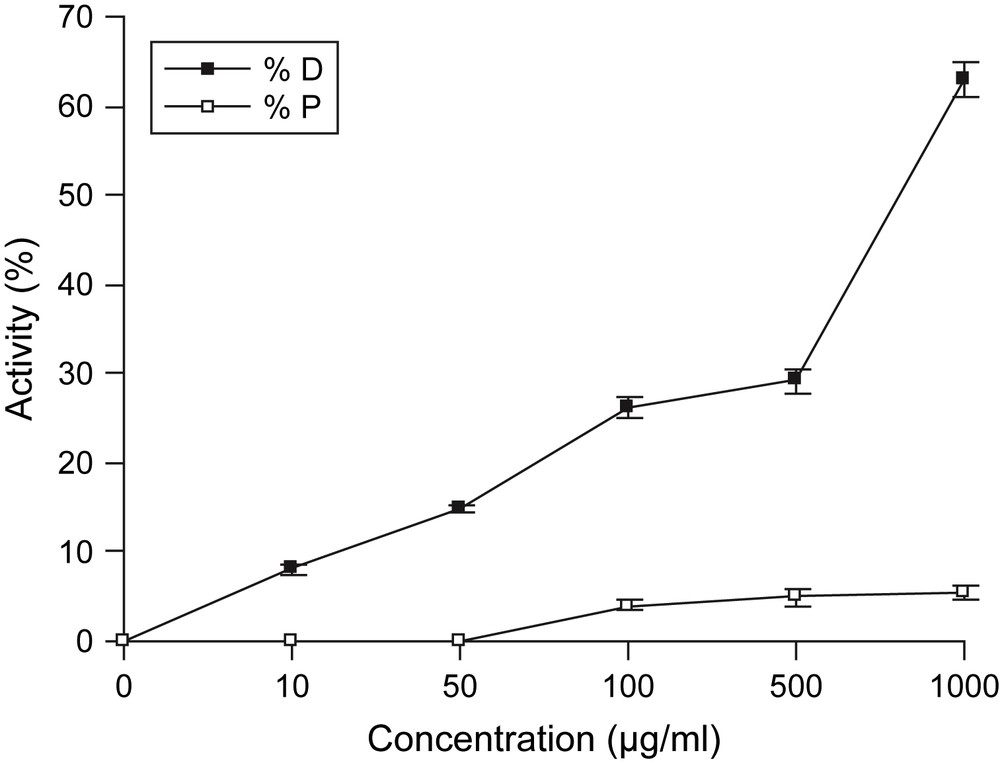
Cytotoxicity and anti-HSV-1 activity of the essential oil of C. trifurcatum flowerheads. This figure shows the antiviral and cytotoxic activities of the essential oil of C. trifurcatum flowerheads tested at various concentrations, after incubation for 72 h. Vero cells infected by HSV-1 (MOI 0.001 ID50/ml) were mammalian fibroblastic cells and HSV-1 was H. simplex virus type 1. Antiviral activity was expressed as the percentage of protection of virus-infected cells (% Protection P: □). Cytotoxic activity was observed as the percentage of destruction of mock-infected cells (% Destruction D: ■). The 50% cytotoxic (CC50) oil concentration was 735.9 μg/ml and the 50% effective (EC50) oil concentration was more than 1000 μg/ml. Values are presented as mean ± s.e.m. of three replicates. Masquer
Cytotoxicity and anti-HSV-1 activity of the essential oil of C. trifurcatum flowerheads. This figure shows the antiviral and cytotoxic activities of the essential oil of C. trifurcatum flowerheads tested at various concentrations, after incubation for 72 h. Vero cells infected ... Lire la suite
4 Conclusion
In our study it has become clear that essential oil of flowerheads of C. trifurcatum has a great potential to inhibit the growth of S. epidermidis and B. subtilis along with other bacteria tested. However, it was not active against the H. simplex virus of type 1 and it was generally tolerated by Vero cells. Analysis by gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS) demonstrated that this oil contained mainly terpenoid compounds and that it was exceptionally rich in monoterpenes. We have also characterized the C. trifurcatum from Tunisia (Zeramdine) as the chemotype limonene (20.89%), γ-terpinene (19.13%) and 1,8-cineole (10.64%). These bioactivities and chemical composition of the essential oil were reported here for the first time. However, it was still necessary to investigate in vivo bioactivity and toxicity of this oil and its major constituents.


