1 Introduction
Polyaromatic polymers, like Poly-Ether-Ether-Ketone (PEEK) and Poly-Phenyl-Sulfone (PPSU), are very well known high-temperature engineering resins [1]. Their unique combination of chemical and physical properties, such as high hydrolytic, thermal and oxidation stabilities, low inflammability, and excellent mechanical properties, is united with low cost and an easy processability [2]. The high versatility of these fully aromatic polymers allows them to be used for a wide range of aerospace, medical, electronic and industrial applications [3,4]. Chemical functionalization of these polymers may lead to further innovative applications. Sulfonation, for instance, adds proton conductivity and improves their solubility, making polyaromatic macromolecules suitable for use in polymer electrolyte membrane fuel cells (PEMFCs) [5,6].
Among the several chemical modifications of the aromatic backbone, the formation of organic/inorganic Class II nano-hybrids seems to be very promising [7]. They are an interesting class of polymeric materials possessing a structural flexibility suitable for different applications; such materials, in fact, can exhibit properties intermediate between those of the organic and inorganic components [8]. Recently, we reported different methods for the functionalization of polyarylene systems with the aim of developing new polymeric membranes for fuel cells especially based on PEEK and PPSU [9–12]. The synthetic approach for the functionalization is different for the two polymers. In the case of PEEK, the common organic reactions are not easy to carry out given its insolubility in almost all solvents, probably due to the strong interaction among the polymeric chains [13]. However, its sulfonated derivative shows an improved hydrophilicity that allows conducting reactions in homogeneous conditions. PPSU, instead, is soluble in a few organic solvents such as tetrahydrofuran (THF) and dichloromethane.
In the next section, we will describe the different synthetic approaches followed in the preparation of polymeric membranes for fuel cell applications. Mainly two classes of reactions will be reported: the sulfonation of aromatic backbones and the functionalization via metalation reactions.
2 Experimental
2.1 Materials
Poly-Ether-Ether-Ketone (Victrex, PEEK, 450 PF, MW = 38,300), Poly-Phenyl-Sulfone (Solvay, PPSU, MW = 46,173), HSO3Cl (Fluka) and all other chemicals (Aldrich) were of reagent grade and were used as-received. Anhydrous THF and CH2Cl2 were prepared according to literature procedures [14].
2.2 Sulfonation of PEEK and PPSU
The degree of sulfonation (DS) was evaluated by 1H NMR [15], titration or by Elemental Analysis.
2.2.1 Sulfonation with H2SO4
In a typical procedure, the polymers were added to concentrated H2SO4 (96%). The mixture was kept under stirring at the selected temperature (see Table 1 for concentration, temperature, time), then it was poured into ice-cold water. The precipitate was filtered, washed with water to neutral pH and dried under vacuum for 5 h [9].
| Substrate | Sulfonating agenta (N) | Temperature (°C); time (h) | Solvent | Degree of sulfonation |
| PEEK | H2SO4 (0.07) | 25; 70 | – | 0.54 |
| 25; 100 | – | 0.60 | ||
| 25; 170 | – | 0.70 | ||
| 50; 120 | – | 0.90 | ||
| PPSU | H2SO4 (0.25) | 50; 5 | – | 1.80 |
| 50; 15 | – | 2.0 | ||
| PEEK | HSO3Cl (0.87) | 50; 5 | – | 0.80b |
| PPSU | HSO3Cl (0.03) | Reflux; 48 | CH2Cl2 | 1.80 |
| PPSU | ClSO3Si(CH3)3 (0.03) | Reflux; 48 | CH2Cl2 | 0.05 |
| Reflux; 168 | CH2Cl2 | 1.20 |
a Concentration is expressed as equivalent of polymer per liter of solution.
b In this condition, the reaction affords DS = 0.8 and DCL (degree of cross-linking) = 0.2 (see Ref. [12]).
2.2.2 Sulfonation with HSO3Cl
The polymers were added to HSO3Cl and the mixtures were stirred at the selected temperature under nitrogen atmosphere. After 1 h, the polymers were completely dissolved giving viscous orange solutions. After further stirring, the mixtures were cooled to RT and the polymers precipitated by addition of anhydrous CH2Cl2 (100 mL). In the presence of solvent (see Table 1), the initially soluble PPSU becomes progressively insoluble as soon as part of the polymer reacts with the acid. In this case, no further CH2Cl2 was added. The sulfochlorinated polymers were added to a saturated solution of NaHCO3 and stirred at reflux for 5 h. The resulting solutions were treated with 6 M H2SO4 until complete precipitation (pH = 2). The products were filtered, washed with water to neutral pH and dried under vacuum for 5 h [11].
2.2.3 Sulfonation with ClSO3Si(CH3)3
PPSU was added, under nitrogen, to anhydrous CH2Cl2. The yellow solution was stirred at reflux for 1 h, then a solution of ClSO3Si(CH3)3 in anhydrous CH2Cl2 was added dropwise. After 5 h, a light brown precipitate separated from the solution. The mixture was kept at reflux for a total of 216 h. The product was filtered, washed with water to neutral pH and dried under vacuum for 5 h [12].
2.3 Silylation of PEEK
In a typical procedure, S–PEEK (3 × 10−3 N) was dissolved under nitrogen in DMSO/THF 1:25 solution. The resulting solution was cooled to −60 °C, then an excess of BuLi (about 10:1) and N,N,N′,N′-tetramethylethylenediamine (TMEDA, 1:1 with BuLi) were added, and the solution was stirred for 8 h at −60 °C. Alkyl- or aryl-silane (RSiX3, in the selected ratio with BuLi) was then added and the solution was slowly warmed to room temperature, then kept at reflux overnight. Absolute ethanol was added, maintaining the mixture at reflux for further 5 h. After cooling to RT, the precipitate formed was left to settle overnight, then filtered and washed with cold water until no chlorides were present. The products were dried under vacuum for 4 h. The degree of silylation (DSi) was evaluated by Elemental Analysis [9,12].
2.4 Silylation of PPSU
PPSU (5 × 10−2 N) was added, in a nitrogen atmosphere, to anhydrous THF. The solution was stirred at room temperature for 1 h, then cooled to −60 °C and kept at this temperature for 1.5 h. An excess of BuLi (about 2:1) and TMEDA (1:1 with BuLi) were then added and the solution was stirred for 2 h at −60 °C. After this time, alkyl- or aryl-silane (RSiX3, 0.1:1 with BuLi) was added and the solution was slowly warmed to room temperature, and then kept at reflux for 2 h. After cooling to RT, the formed precipitate was filtered and washed with water until no chlorides were detected. The products were dried under vacuum for 6 h. The degree of silylation (DSi) was evaluated by Elemental Analysis [11,16].
2.5 Synthesis of sodium difluoro [(phenyl)(hydroxy)(PPSU)-silyl]-oxy acetate (compound 1)
The phenylsilanol–PPSU derivative (0.543 mequiv), obtained from the above reaction using a PPSU:PhSiCl3 ratio of 0.07 (DSi = 0.07), was dispersed in CH3OH (53 mL), then CH3O−Na+ (0.27 mmol) was added and the mixture was kept under stirring for 2 days. After this time, CF2ClCO2−Na+ (0.27 mmol) was added and the reaction was refluxed for 6 days. After cooling to RT, the precipitate was filtered and washed with methanol. The product was dried at 60 °C for 12 h. The degree of silylation (DSi) was evaluated by Elemental Analysis. The product was characterized by 1H and 13C NMR spectroscopies.
2.6 Structural and thermal characterizations
1H and 13C NMR spectra were recorded with a Bruker Avance 400 spectrometer operating at 400.13 and 100.56 MHz, respectively. Non-deuterated DMSO and dimethylacetamide (DMAc) were used as solvent with D2O as external lock. Chemical shifts (ppm) are referenced to tetramethylsilane (TMS). Thermogravimetric analyses were carried out using a thermobalance (STA 409, Netzsch) in Ar flow (80 mL/m) with a heating rate of 10 °C/min in the range 25–800 °C.
3 Results and discussion
The polyarylether ketones or sulfones (Fig. 1) are a class of polymers consisting of sequences of 1,4-disubstituted phenyl rings separated by ether (–O–) and carbonyl (–CO–) or sulfonyl (–SO2–) linkages. Being fully aromatic, these polymers have excellent thermal oxidation resistance with a high glass transition temperature. PEEK is semi-crystalline and a flexible polymer, PPSU is amorphous and more rigid, because of the direct linkage between the two aromatic rings.
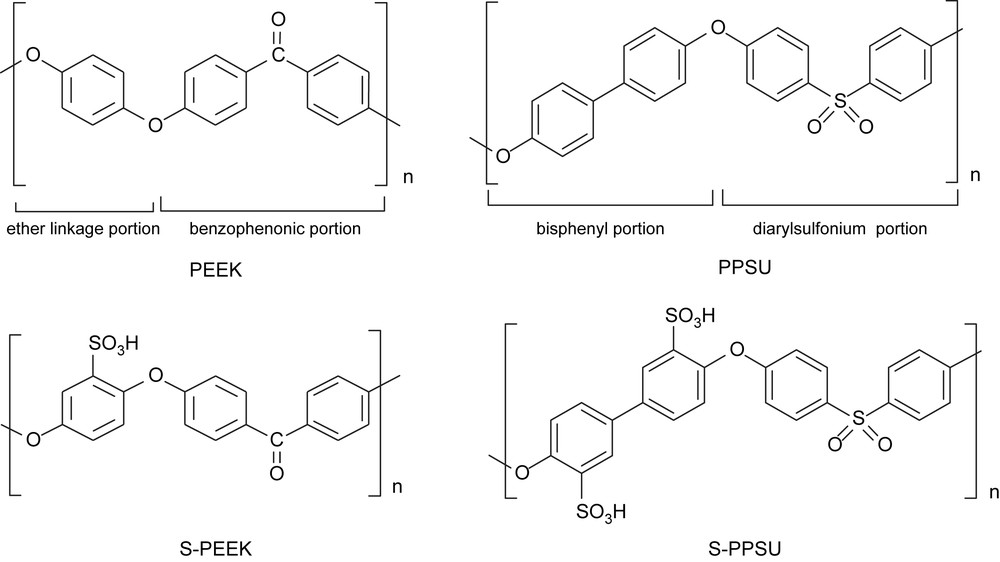
Repeat units of PEEK, PPSU, S–PEEK and S–PPSU.
Their good oxidation resistance is correlated to the presence of only sp2 aromatic carbon–hydrogen bonds that have typical bond energy around 435 kJ mol−1, compared with sp3 aliphatic C–H bonds that have energy around 350 kJ mol−1. Both these polymers must be sulfonated to achieve the conducting properties necessary to function in a fuel cell. The introduction of a sulfonated group is also necessary to improve solubility properties, especially in the case of PEEK.
3.1 Post-sulfonation of PEEK and PPSU
Sulfonation of aromatic compounds is an electrophilic substitution reaction (SEAr) and represents a powerful and versatile tool for synthetic purposes. When applied to macromolecules, it is generally called post-sulfonation reaction, because it occurs on the formed polymer [17]. Aryl sulfonation is known to be a reversible reaction, but below 100 °C and with a high concentration of the electrophilic species, the constant of sulfonation is much higher than the constant of de-sulfonation [17]. The application of this reaction depends on the substituents present in the ring. In PEEK, only the phenyl ring between the two ether links can be sulfonated under relatively mild conditions. The product is called S–PEEK (Fig. 1). This phenyl ring has four equivalent ortho positions for sulfonation, but at most one sulfonic group per repeat unit can be introduced with this procedure. In fact when one of the four ortho hydrogens undergoes the substitution by effect of the sulfonating agent, the ring becomes deactivated because of the electron-withdrawing effect of the sulfonic group and further sulfonation does not take place. The other two phenyl rings, linked to a carbonyl group, are deactivated. In the case of PPSU, the presence of two activated phenyl rings allows the introduction of two sulfonic groups per repeat unit. The product is called S–PPSU (Fig. 1).
Different sulfonating agents can be employed: concentrated sulfuric acid, chlorosulfonic acid, sulfur trioxide, sulfur trioxide–triethyl phosphate complex, trimethylsilyl chlorosulfonate. Different procedures bring a different amount of sulfonated products [18–22]. The amount of sulfonic groups in the polymer can be expressed as degree of sulfonation (DS), where DS is the molar ratio of sulfonic groups per monomer unit of polymer, or as Ion Exchange Capacity, which corresponds to the milliequivalents of ionic groups per gram (mequiv/g or mmol/g) of polymer. The extent of sulfonation can be controlled by reaction time, acid concentration, and temperature (DS ≤ 1 for PEEK and ≤2 for PPSU). We performed different sulfonation reactions in different conditions as reported in Table 1.
The sulfonation reaction carried out in chlorosulfonic acid, a strong reagent, allows the introduction of the acidic moieties, but also permits to introduce some degrees of cross-linking among the chains via SO2 groups. In this acidic medium, the benzenesulfonic acid, formed during the sulfonation of the polymer, can again react with chlorosulfonic acid to form an intermediate pyrosulfonate (see Scheme 1). The electrophilic pyrosulfonate attacks the aryl ring giving a cross-linked structure. In 96% H2SO4, this reaction does not occur as the water present in the acid decomposes the intermediate pyrosulfonate [19]. The use of a mild reagent like ClSO3Si(CH3)3 allows a careful control of the degree of sulfonation.
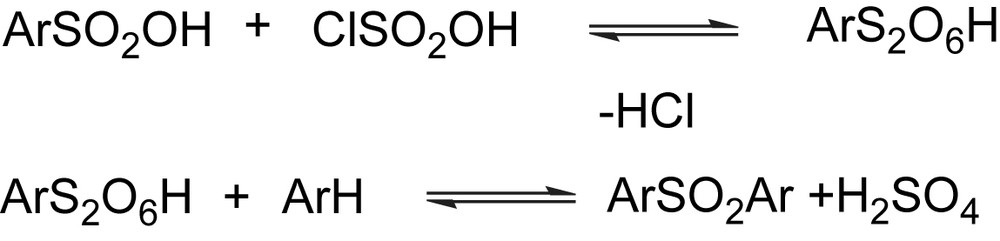
3.2 Functionalization via metalation reactions
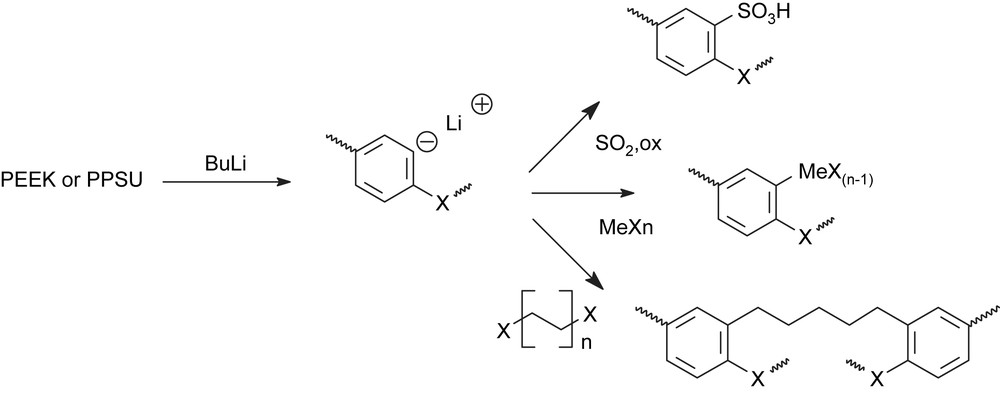
Among the different possibilities to form a metal carbon bond, one of the more promising is the generation of an organolithium compound by hydrogen metal exchange. The exchange reactions are particularly favorable when the new organometallic is stabilized by resonance. The position of the metalation is not only determined by the relative acidity of the hydrogens, but also by the directing effect of present substituting group. Sulfonyl groups, which can complex the metal atom, have a great influence on the position of lithiation [17,9]. In general, metalation reactions allow functionalizing the benzophenonic or benzenesulfone ring. The generated anionic species can be used as precursor in several reactions, for example, for the introduction of sulfonated groups on the deactivated ring or for the generation of a C–metal bond or for the formation of a cross-linking between two units (Scheme 2).
In the past, we used the metalation procedure to synthesize C–Si derivatives of S–PEEK and S–PPSU (named SOSi–PEEK and SiS–PPSU, Fig. 2) by reaction with alkyl- or aryl-silane derivative. The synthetic strategy was chosen on the basis of the solubility properties of the two polymers. PEEK is insoluble in most solvents, while its sulfonated product is soluble in few solvents, for example DMSO and THF. Therefore, the synthetic strategy foresaw first the sulfonation of the polymer, its purification and then the silylation. A different approach was used when the sulfonation reaction was carried out in chlorosulfonic acid. The silylation step was performed in situ due to the solubility of the chlorosulfonate derivatives in dichloromethane. In the case of PPSU, being soluble in organic solvents, the reaction steps were inverted, conducting first the silylation and then the sulfonation. Fig. 2 shows 1H spectra of two silylated compounds [12].
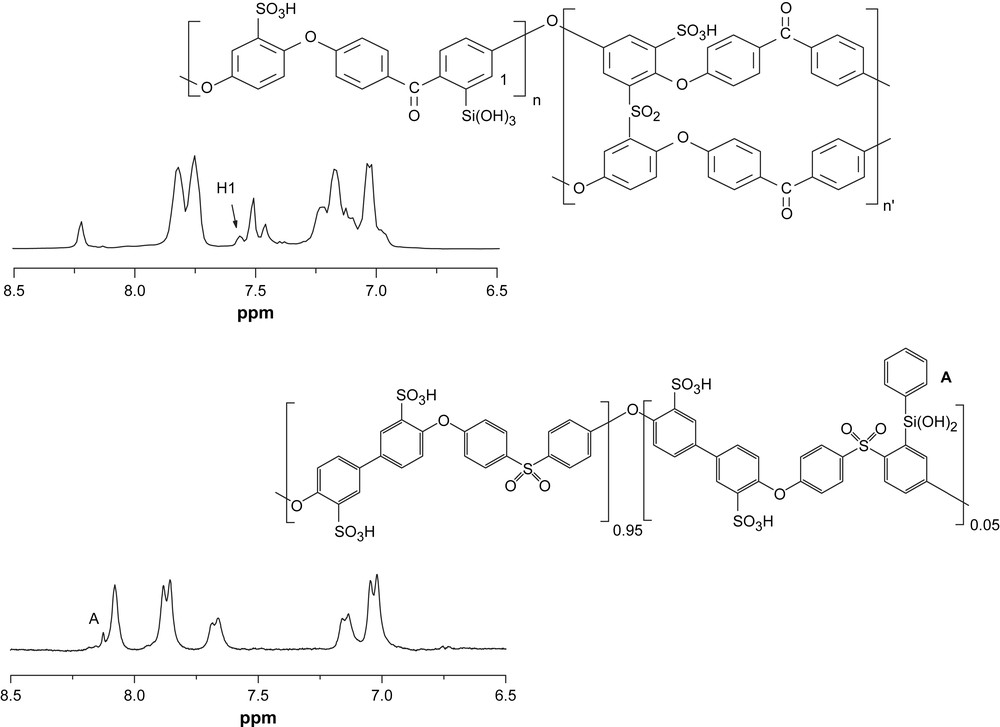
1H NMR spectra of (a) SOSi–PEEK (b) SiS–PPSU.
A different application of this reaction is the synthesis of compound 1. Scheme 3 shows the reaction pathways. In this case, the phenylsilanol derivative acts, in basic conditions, as nucleophile on the chloro-difluoro-acetate, leading to a product containing an acidic functionality. Fig. 3 shows the 13C NMR spectrum of compound 1 compared to the spectrum of PPSU [23], it is possible to distinguish the peaks due to the acetate group. To obtain the appropriate proton conductivity for fuel cell membranes, it is necessary to increase the degree of sulfonation (DS) of PPSU up to values greater than 1. It is known that in these systems a high degree of sulfonation leads to a decrease in mechanical properties and swelling of the membrane [24,25]. The functionalization of polyarylsulfones with perfluoro aliphatic groups, terminating with acidic moieties, instead of the functionalization with sulfonic groups linked directly to the aromatic ring, can lead to better hydrophobic properties for the organic backbone while assuring proper conductivity. Furthermore, the presence of a pendant group should increase the chain mobility responsible for conduction. Studies on the development of such proton-conducting polymers are currently in progress.

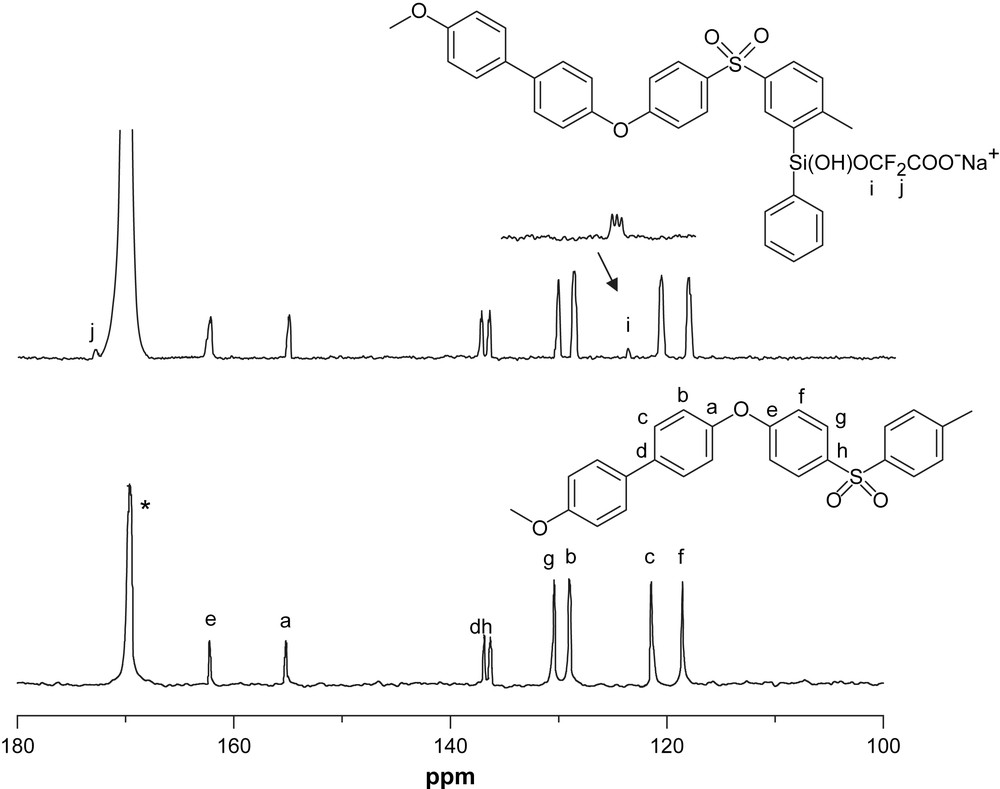
13C NMR spectra of compound 1.
3.3 Thermal stability of hybrid polymers
In Fig. 4, thermogravimetric analysis of some selected hybrid polymers is reported. The remarkable thermal stability of PEEK is confirmed and thermolysis observed near 500 °C. Sulfonation leads in all cases to a reduction of thermal stability. Loss of sulfonate groups is already observed below 200 °C for SO–PEEK with final pyrolysis below 400 °C. Silylation stabilizes the macromolecules, as exemplified by SOSi–PEEK, where sulfonate loss and pyrolysis are observed at nearly 100 °C higher temperatures. This example shows the large potential of a synthetic strategy playing on the relative amount of sulfonation and metalation reactions on polyaromatic polymers.
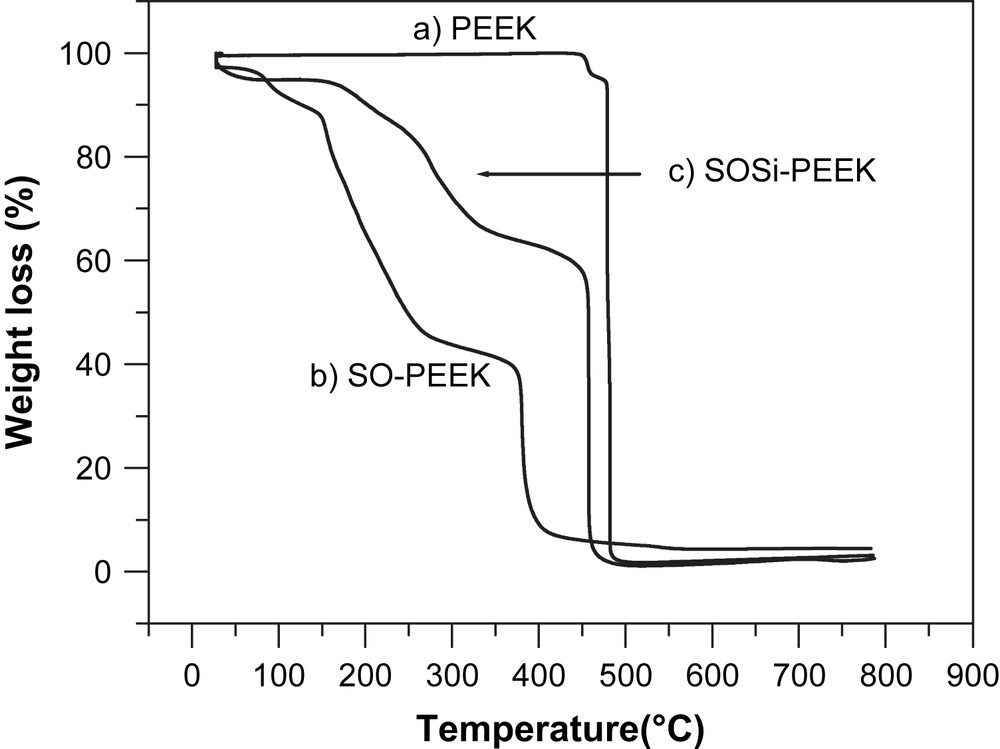
Thermogravimetric curves for (a) PEEK, (b) SO–PEEK, (c) and SOSi–PEEK.
4 Conclusion
By combining sulfonation and silylation reactions of PEEK and PPSU polymers under appropriate experimental conditions, one can achieve proton conducting, thermally and mechanically stable hybrid polymer membranes. Whereas a high degree of sulfonation improves the proton conductivity, but reduces the thermal and mechanical stabilities, leading to membrane swelling and even solubility in water; silylation improves significantly thermal and mechanical stabilities. The outlined chemical synthesis approach appears promising for the development of hybrid membranes meeting the operative requirements of intermediate temperature PEM fuel cells.
Acknowledgment
E.S. gratefully acknowledges a thesis grant from Franco-Italian University (Vinci program 2006).


