1 Introduction
Aromatic polyfunctionalized compounds present a wide scope of applications not only in the pharmaceutical field but also in agrochemicals and flavour and fragrance chemistry [1,2]. Their synthesis still represents an actual challenge, and the allylation of aromatic rings through the Friedel–Crafts reaction is of great significance in view of laboratory and industrial functionalization. For example, eugenol and anetol are, respectively, characteristic of the flavour of cloves [3] and of the French drink called Pastis. The synthesis of many biologically active molecules, such as vitamin K1 [4] or co-enzyme Q1 [5], could involve a Friedel–Crafts allylation. Unluckily, the most extensively utilized Friedel–Crafts allylation reaction has proved unsatisfactory [6]. The standard Friedel–Crafts allylation conditions employ an allyl halide and a Lewis acid mediator such as aluminium (III) chloride. Thus, hydrogen halide is coproduced and can induce side reactions. The reaction can occur both at the double bond and at the allylic carbon, so that polyaryl-substituted alkanes can be observed. Aluminium hydroxide is excreted by usual work up so that a secondary product is generated, whose destruction must be taken into account. Finally, the yield of the desired allylated product is generally low.
The Friedel–Crafts allylation reaction has undergone recent important improvements with the advent of heterogeneous catalysis [7–10]. An additional effort has been carried out to decrease the amount of the promoter, from a stoichiometric to a catalytic quantity. Moreover, some attention has been devoted to catalytic reactions that use allylic alcohols or acetates instead of organic halides, e.g. which do not produce halogen-containing by-products [11–16].
Interesting results have been obtained with Lewis acids. For example, boron trifluoride has been able to catalyse regio- and stereospecific alkenylation of phenolic ethers with prenyl and geranyl diisopropyl phosphates [17], without any appreciable side reactions. Scandium triflate (10 mol%) has been reported to efficiently catalyse the allylation of activated aromatic derivatives in good yields, using the corresponding allylic alcohols as the alkylating agents [18,19]. Catalytic Friedel–Crafts allylation reaction of toluene has been achieved with allyl silyl ethers with 10 mol% of Cl2Si(OTf)2 or Hf(OTf)4 [20]. Metal bis(trifluoromethylsulfonyl)amides, or metallic triflimidates, have, to our knowledge, not yet been described as catalysts for the Friedel–Crafts allylation.
In the present work, the activity of zinc triflimidate, Zn(NTf2)2, is compared with that of other metal triflates and triflimidates, in the Friedel–Crafts allylation of aromatic derivatives.
2 Results and discussion
Our attention was first focused on the reactivity of anisole 1a and isoprenyl acetate 2a (Eq. (1)) as a model allylation reaction. Our efforts were dedicated to the search for experimental conditions leading selectively to the mono-allylated products 3a and 4a.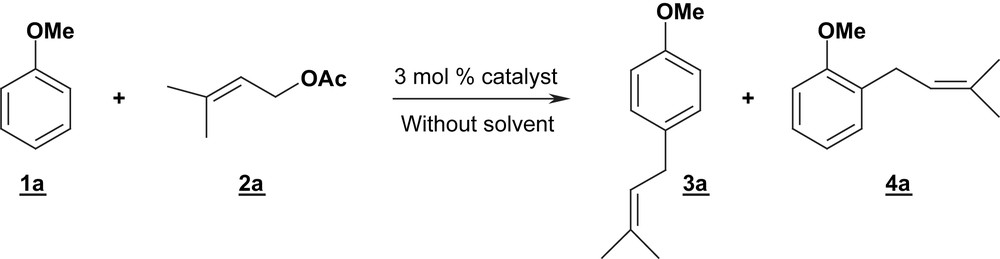
The activity of various Lewis acids derived from metallic triflates catalysts was investigated in detail. Different triflate or triflimidate salts were prepared following a reported electrochemical procedure [21]. The reaction was monitored by gas chromatography and stopped after complete consumption of isoprenyl acetate 2a. Preliminary tests between anisole 1a with isoprenyl acetate 2a (ratio 1a:2a = 10:1) proved unsuccessful when performed in the presence of 3% molar of Fe(II), In(III), Sn(IV) or Al(III) triflimidates. Cu(II) and W(VI) catalysts led to yields of 5–16% for 3a/4a (Table 1, entries 1–2), the bis-arylated products being the major compounds. The best results were obtained in the presence of 3 mol% of Zn(II) or Ni(II) triflimidates, at 100 °C, without added solvent, affording the mixture of two isomers 3a/4a with 93 and 86% yields, respectively (Table 1, entries 4 and 7). The ortho/para assignments were determined by NMR and the ortho/para ratios were calculated by GC:para isomers were always obtained as the major products. With Zn(NTf2)2 and Ni(NTf2)2 at 80 °C, the reaction rates decreased and incomplete conversions were observed, resulting in lower yields (Table 1, entries 3 and 6). At higher temperature (120 °C), a rapid and complete conversion of the isoprenyl acetate 2a occurred, but a moderated yield of 3a/4a seemed to indicate partial polymerization (Table 1, entries 5 and 8).
Influence of the catalyst and the temperature in the allylation reaction of Friedel–Crafts (ratio 1a:2a = 10:1, 3% molar of catalyst, without added solvent).
| Entry | Catalyst | Temperature (°C) | Reaction time | Yield of 3a + 4aa (%) | Ratio 3a/4ab |
| 1 | Cu(NTf2)2 | 120 | 2 h | 5 | 3.8/1 |
| 2 | W(NTf2)6 | 120 | 5 h | 16 | 2.7/1 |
| 3 | Zn(NTf2)2 | 80 | 6 h | 66c | 2.9/1 |
| 4 | Zn(NTf2)2 | 100 | 3 h | 93d | 2.8/1 |
| 5 | Zn(NTf2)2 | 120 | 2 h | 46 | 2.4/1 |
| 6 | Ni(NTf2)2 | 80 | 24 h | 55c | 2.8/1 |
| 7 | Ni(NTf2)2 | 100 | 3 h | 86d | 2.6/1 |
| 8 | Ni(NTf2)2 | 120 | 2 h | 52d | 2.3/1 |
| 9 | Zn(BF4)2 | 120 | 4 h | 62 | – |
| 10 | Ni(OTf)2 | 120 | 45 min | 47d | 2.4/1 |
a Yields calculated by NMR after extraction and isolation of the crude product.
b The para/ortho assignments were determined by NMR and the ratios calculated by GC.
c Uncomplete conversion of allyl acetate 2a.
d Isolated yield of 3a + 4a after distillation.
The nature of the counter-ion proved to be essential for the efficiency of the catalytic system. The catalytic activity of various zinc and nickel salts was examined in detail. Among them, NiCl2, NiBr2 and Ni(BF4)2, used in 3 mol%, failed to provide the corresponding coupling products. When ZnCl2, ZnBr2 and Zn(OTf)2 were used as the catalysts, the expected allylated compounds were formed in less than 20% yield, and bis-arylated derivatives were obtained. Zn(BF4)2 and Ni(OTf)2 were able to catalyse the coupling reaction, leading to 3a/4a, with 47 and 62% yields, respectively (Table 1, entries 9 and 10). Zn(NTf2)2 presented the higher catalytic activity, allowing the formation of ortho and para isomers 3a/4a with high selectivity.
The influence of the 1a:2a ratio was changed from 10:1 to 1:1. When the ratio 1a:2a decreased, the bis-allylated products were favoured. With a 1:1 ratio, bis-allylation of anisole occurred almost quantitatively. The best results, concerning the yield of 3a/4a, were observed with a 10:1 ratio.
We then examined the influence of the solvent. In CH3NO2, no major changes occurred in yields or selectivity, when compared to the same conditions without solvent.
To extend the scope of the reaction, various allyl acetates were reacted with anisole 1a, in the presence of 3 mol% of Zn(NTf2)2, at 100 °C, without solvent, with a 1a:2a ratio of 10:1 (Table 2, entries 1–3). Cinnamyl acetate 2b afforded the mono-allylated adducts 3b/4b in 63% isolated yield, with a para to ortho ratio of 6.4. Low conversion was observed with crotyl acetate 2c, leading to the expected products 3c/4c in 16% yield, though a selectivity towards the mono-allylated compounds higher than 80%. Methallyl acetate and allyl acetate produced low yields of the expected products, even after 24 h of reaction.
Allylation of aromatic compounds with allyl derivatives (ratio 1:2 = 10:1) with 3 mol% of Zn(NTf2)2 as the catalyst, without added solvent, at 100 °C.
| Entry | Aromatic derivative | Allyl derivative | Reaction time | Yield of 3 + 4a (%) | Isolated compounds Ratiob para/ortho |
| 1 | 3 h | 93 | 3a/4a 2.8/1 | ||
| 2 | 1a | 5 h | 63 | 3b/4b 6.4/1 | |
| 3 | 1a | 24 h | 16 | 3c/4c -c | |
| 4 | 1a | 3 h | <10d,e | 3a/4a 3.1/1 | |
| 5 | 1a | 3 h | 45 | 3a/4a 2.6/1 | |
| 6 | 2a | 2 h | 87 | ||
| 7 | 2a | 2 h | 57 | ||
| 8 | 2a | 2 h | 74d | 3f/4f 6.9/1 | |
| 9 | 2a | 2 h | 90 |
a Isolated yield of 3 + 4 after distillation under reduced pressure.
b The para/ortho assignments were determined by NMR and the ratios calculated by GC.
c Not determined.
d Yields calculated by NMR.
e Conversion rate of allyl derivative 2 lower than 100%.
These experiments pointed out an important difference in reactivity between differently substituted allylic substrates. Indeed, the reaction occurred efficiently with tri-substituted olefins such as 2a or with electron-rich double bonds such as in 2b. On the other hand, mono- (allyl acetate) or di-substituted olefins (2c, methallyl acetate) revealed low allylation efficiency. These results strongly support a carbocationic-type mechanism, favouring the reaction with the more electron-rich olefins.
In order to evaluate the influence of the leaving group in allyl substrates 2, the allylation reaction was examined with isoprenyl bromide 2d (Table 2, entry 4) and isoprenyl alcohol 2e (Table 2, entry 5). A low conversion was observed when anisole 1a was reacted with allyl bromide 2d. In contrast, the reaction of anisole 1a and isoprenyl alcohol 2e was complete after 3 h, affording a mixture of 3a/4a in 45% yield.
The allylation with prenyl acetate was further extended to differently substituted electron-rich aromatic compounds (Table 2, entries 6–9). Whatever the nature of the activating group (OMe, OR, CH3), the mono-allylation occurred selectively and with good yields. The allylation of p-methoxyanisole 1c afforded the 2-prenyl derivative 4e as sole product, with 57% yield (Table 2, entry 7). Toluene 1d led to the corresponding mixture of ortho and para isomers 3f/4f with 74% yield and high para selectivity (Table 2, entry 8). For veratrole 1b and 1,3-benzodioxole 1e, a single isomer was formed in each case, with 87 and 90% yields, respectively (Table 2, entries 6, 9). In the case of bromobenzene, no conversion was observed after 24 h, with either zinc(II), nickel(II) or copper(II) triflimidates.
3 Conclusions
In conclusion, the use of zinc bis(trifluoromethylsulfonyl)amide as a Lewis superacid catalyst was shown to be efficient for Friedel–Crafts allylation processes. The main features of the method are the low catalyst loading (3 mol%) and the mild reaction conditions. The procedure is well adapted to activated aromatic rings and prenyl and cinnamyl acetates. The reaction affords the mono-allylated isomers with good yields and with high para selectivities. Further extension of this methodology is currently under investigation and further studies on the mechanism of this reaction will be reported in due course.
4 Experimental section
All the reactions were carried out under nitrogen atmosphere. Aromatic and allylic compounds were purchased from Aldrich and Alfa Aesar. Flash column chromatographies were performed using silica gel (40–63 μm, VWR). Analytical thin-layer chromatographies were carried out using aluminum plates pre-coated with 40–63 μm silica gel and impregnated with a fluorescent indicator (254 nm). NMR spectra were measured in CDCl3 on a Brucker AC 200 FT spectrometer (200 MHz), at room temperature, with TMS as an internal reference. The 1H chemical shifts were reported as parts per million downfield from tetramethylsilane (TMS), and the 13C chemical shifts were referenced to the solvent peak CDCl3 (77.16 ppm). Mass spectra were measured on a Gas Chromatograph Agilent 6890L coupled by a mass detector Agilent 5973 N. Mass spectra were obtained by electron ionisation at 70 eV of the mass range of 35–400. Products were characterized by 1H, 13C, DEPT135 NMR and mass spectra.
4.1 Preparation of the catalysts
Some catalysts were purchased from Aldrich, VWR or Riedel de Haën: ZnCl2 (Aldrich: 20,808-6), ZnBr2 (Riedel de Haën: 02128), Zn(BF4)2 (Aldrich: 333875), ZnOTf2 (Aldrich: 290068), NiCl2 (Aldrich: 451193), NiBr2 (Aldrich: 449156), Ni(BF4)2 (VWR: ALFAB20334.14). The other triflate (Ni(OTf)2) or triflimidate (Zn(NT2)2, Ni(NTf2)2, Cu(NTf2)3, W(NTf2)6) salts were prepared following a reported electrochemical procedure [21].
4.2 General allylation procedure: synthesis of 3a/4a, 3b/4b, 3c/4c, 3d, 4e, 3g
Aromatic compound 1 (10 mmol) and allyl acetate 2 (1 mmol) were stirred with the catalyst (3 mol%), without added solvent, at 100 °C, under nitrogen atmosphere. The reaction was monitored by GC and generally stopped after complete consumption of 2. After reaction, the mixture was washed with 10 mL of HCl 1 N. Then organic layer was washed with 10 mL of water. The crude product was concentrated under reduced pressure, leading to compounds 3 and 4 together with some by-products. Distillation under reduced pressure allowed mono-allylated compounds 3a/4a, 3b/4b, 3c/4c, 3d, 4e, and 3g to be isolated and characterized.
1-Methoxy-4-(3-methylbut-2-enyl)benzene, 3a [22]: colorless oil; bp 69 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.72 (d, 4J = 1.5 Hz, 3H, CH3), 1.74 (d, 4J = 1.5 Hz, 3H, CH3), 3.29 (d, 3J = 7.4 Hz, 2H, CH2), 3.79 (s, 3H, OCH3), 5.31 (tqq, 3J = 7.4 Hz, 4J = 1.5 Hz, 1H, (CH3)2CCH), 6.84 (d, 3J = 8.6 Hz, 2H, Ar), 7.10 (d, 3J = 8.6 Hz, 2H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 17.3, 25.4, 33.0, 54.9, 113.4, 123.2, 129.2, 131.7, 133.5, 157.8. MS (70 eV, m/z, %): 176 (52) [M+], 161 (100), 121 (38), 91 (95), 77 (72), 65 (50).
1-Methoxy-2-(3-methylbut-2-enyl)benzene, 4a [23]: colorless oil; bp 69 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.64 (d, 4J = 1.5 Hz, 3H, CH3), 1.66 (d, 4J = 1.5 Hz, 3H, CH3), 3.22 (d, 3J = 7.4 Hz, 2H, CH2), 3.75 (s, 3H, OCH3), 5.26 (tqq, 3J = 7.4 Hz, 4J = 1.5 Hz, 1H, (CH3)2CCH), 6.8–7.2 (m, 4H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 17.6, 25.6, 33.3, 55.3, 110.1, 120.3, 123.5, 127.7, 129.0, 131.4, 134.4, 158.2. MS (70 eV, m/z, %): 176 (22) [M+], 161 (27), 121 (17), 91 (100), 77 (39), 65 (35).
1-Methoxy-4-(3-phenylprop-2(E)-enyl)benzene, 3b [24]: colorless oil; bp 92 °C (0.1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 3.42 (d, 3J = 5.6 Hz, 2H, CH2), 3.71 (s, 3H, OCH3), 6.26 (dt, 3J = 15.8 Hz, 3J = 5.6 Hz, 1H, CH–CH2), 6.36 (d, 3J = 15.8 Hz, 1H, CH–ϕ), 6.78 (d, 3J = 8.8 Hz, 2H, Ar), 7.10 (d, 3J = 8.8 Hz, 2H, Ar), 7.05–7.35 (m, 5H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 38.8, 55.7, 114.3, 126.6, 127.5, 128.9, 130.0, 130.1, 131.2, 132.6, 137.9, 158.3. MS (70 eV, m/z, %): 224 (100) [M+], 209 (22), 193 (33), 165 (22), 121(34), 115 (78), 91 (29).
1-Methoxy-2-(3-phenylprop-2(E)-enyl)benzene, 4b [25]: colorless oil; bp 92 °C (0.1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 3.44 (d, 3J = 5.6 Hz, 2H, CH2), 3.77 (s, 3H, OCH3), 6.24 (dt, 3J = 15.8 Hz, 3J = 5.6 Hz, 1H, CH–CH2), 6.36 (d, 3J = 15.8 Hz, 1H, CH–ϕ), 6.75–6.83 (m, 1H, Ar), 7.05–7.32 (m, 8H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 33.0, 55.7, 110.4, 120.6, 126.5, 127.5, 127.6, 128.9, 129.2, 130.1, 131.2, 132.6, 137.9, 158.3. MS (70 eV, m/z, %): 224 (100) [M+], 209 (28), 193 (63), 165 (28), 115 (93), 91 (77).
1-(But-2(E)-enyl)-4-methoxy-benzene, 3c [26]: colorless oil; bp 59 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.59 (d, 3J = 5.0 Hz, 3H, CH3), 3,17 (d, 3J = 5.0 Hz, 2H, CH2), 3.70 (s, 3H, OCH3), 5.3–5.6 (m, 2H, CHCH), 6.74 (d, 3J = 8.6 Hz, 2H, Ar), 7.01 (d, 3J = 8.6 Hz, 2H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 18.3, 38.6, 55.7, 114.2, 126.4, 129.8, 130.9, 133.9, 158.6. MS (70 eV, m/z, %): 162 (94) [M+], 147 (100), 131 (18), 121 (26), 115 (19), 91 (34), 77 (15).
1-(But-2(E)-enyl)-2-methoxy-benzene, 4c [27]: colorless oil; bp 59 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.60 (d, 3J = 5.0 Hz, 3H, CH3), 3,23 (d, 3J = 6.2 Hz, 2H, CH2), 3.73 (s, 3H, OCH3), 5.3–5.6 (m, 2H, CHCH), 6.7–7.2 (m, 4H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 18.3, 33.4, 55.7, 110.7, 120.9, 127.5, 128.3, 130.0, 130.4, 143.9, 158.6. MS (70 eV, m/z, %): 162 (100) [M+], 147 (79), 131 (30), 115 (26), 91 (57), 77 (13).
1,2-Dimethoxy-4-(3-methylbut-2-enyl)benzene, 3d: colorless oil; bp 94 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.73 (d, 4J = 1.0 Hz, 3H, CH3), 1,76 (d, 4J = 1.0 Hz, 3H, CH3), 3.32 (d, 3J = 7.2 Hz, 2H, CH2), 3.77 and 3,79 (s, 3H, OCH3), 5.22 (tqq, 3J = 7.2 H, 4J = 1.0 Hz, 1H, (CH3)2CCH), 6.3–7.0 (m, 3H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 18.2, 26.1, 34.3, 56.2, 56.3, 110.6, 111.6, 120.5, 123.8, 132.8, 134.8, 147.5, 149.3. MS (70 eV, m/z, %): 206 (97) [M+], 191 (100), 175 (57), 160 (53), 91 (23), 77 (17).
1,4-Dimethoxy-2-(3-methylbut-2-enyl)benzene, 4e [28]: colorless oil; bp 96 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.73 (d, 4J = 1.0 Hz, 3H, CH3), 1.76 (d, 4J = 1.0 Hz, 3H, CH3), 3.32 (d, 3J = 7.4 Hz, 2H, CH2), 3.77 and 3.79 (s, 3H, OCH3), 5.32 (tqq, 3J = 7.4 Hz, 4J = 1.0 Hz, 1H, (CH3)2CCH), 6.5–6.8 (m, 3H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 18.1, 26.2, 28.8, 56.0, 56.4, 110.8, 111.6, 116.4, 122.7, 131.9, 133.4, 152.0, 154.0. MS (70 eV, m/z, %):206 (100) [M+], 191 (57), 175 (28), 160 (12), 151 (20), 121 (18), 91 (13).
5-(3-Methylbut-2-enyl)-1,3-benzodioxole, 3g: colorless oil; bp 87 °C (1 mm Hg); 1H NMR (CDCl3, 200 MHz) (δ, ppm) 1.73 (d, 4J = 1.0 Hz, 3H, CH3), 1.76 (d, 4J = 1.0 Hz, 3H, CH3), 3.32 (d, 3J = 7.2 Hz, 2H, CH2CH), 5.32 (tqq, 3J = 7.2 Hz, 4J = 1.0 Hz, 1H, (CH3)2CCH), 5.97 (s, 2H, O–CH2–O), 6.5–6.8 (m, 3H, Ar). 13C NMR (CDCl3, 50 MHz) (δ, ppm) 18.2, 26.1, 34.4, 101.1, 108.5, 109.2, 121.3, 123.7, 132.9, 136.1, 145.9, 147.9. MS (70 eV, m/z, %) 190 (60) [M+], 175 (20), 145 (100), 135 (15), 117 (33), 77 (15).


