1 Introduction
Vanadium is the key component of many solids which exhibit excellent catalytic properties in redox reactions such as the oxidation of a variety of organic substrates, notably the epoxidation of allylic alcohols. We have shown [1,2] that the use of montmorillonite as a support for the vanadium phase leads to solids with a poor textural porous system. Better porous structures can be found in pillared interlayered clays (PILCs). PILCs are synthesised from the corresponding natural layered mineral, such as montmorillonite, by the replacement of the interlayer cations with positively charged oxohydroxyoligomeric species of the desired pillar-forming cation [3,4]. After calcination, the cationic oligomers turn into oxidic pillars that firmly bind the silicate sheets leading to rigid porous structures open to gases and vapours. Alumina-, titania- and zirconia-pillared clays are among the most widely studied PILCs [5]. Vanadium centres can also be incorporated into the PILC structure [6,7], either during the pillaring procedure or during a post-pillaring treatment.
The aim of this work was to design and synthesise supported vanadium catalysts using alumina-pillared montmorillonite as a carrier. Particular attention was paid to the effect of the preparation procedure on the texture and structure of the resulting solids as well as on the nature of the formed vanadium centres. The materials obtained were tested in the epoxidation of trans-2-hexen-1-ol to 2,3-epoxyhexan-1-ol. The catalytic results are discussed in terms of the physicochemical properties of the samples.
2 Experimental
The starting clay, referred to as Na-mont, was a <2 μm particle-size fraction of natural montmorillonite (CECA, France) in sodium exchanged form. The cation exchange capacity of the clay is 84 meq per 100 g.
Alumina-pillared montmorillonite was prepared following the procedure described by Yamanaka and Brindley [4]. The aluminium hydroxide oligomer solution (OH/Al = 2) was prepared by very slow addition of 0.1 M NaOH solution (500 ml, 0.05 mol) over a 0.1 M AlCl3 solution (250 ml, 0.025 mol) with constant stirring. The resulting solution (75 ml, 5 mmol of Al) was aged at room temperature for 24 h and added to a water suspension of Na-mont (1 g) in 200 ml of ionised water (Al/clay ratio = 5 mmol/g). The mixture was allowed to age for 2 h at 353 K under stirring. The resulting material, denoted as Al-mont, was centrifuged, washed free of chloride ions and dried at 353 K. After calcination in air at 773 K for 5 h the product is referred to as Al-PILC.
Vanadium was introduced by two different methods (Fig. 1). Post-treatment of Al-mont and Al-PILC (1 g) with VCl3/tBuOH solution (5 mmol in 20 ml of tBuOH) led to samples V–(Al-mont) and V–(Al-PILC), respectively. Co-pillaring, that is direct addition of VCl3 in water (0.25 mmol in 20 ml of ionised water) to the aluminium pillaring solution (75 ml, 5 mmol of Al), led to sample (V–Al)-mont. All obtained samples were calcined in air at 573 K. For the sake of comparison, vanadium anchored to the non-pillared montmorillonite, used as a reference in ESR experiments, was obtained by stirring H-mont with VCl3/tBuOH [2].
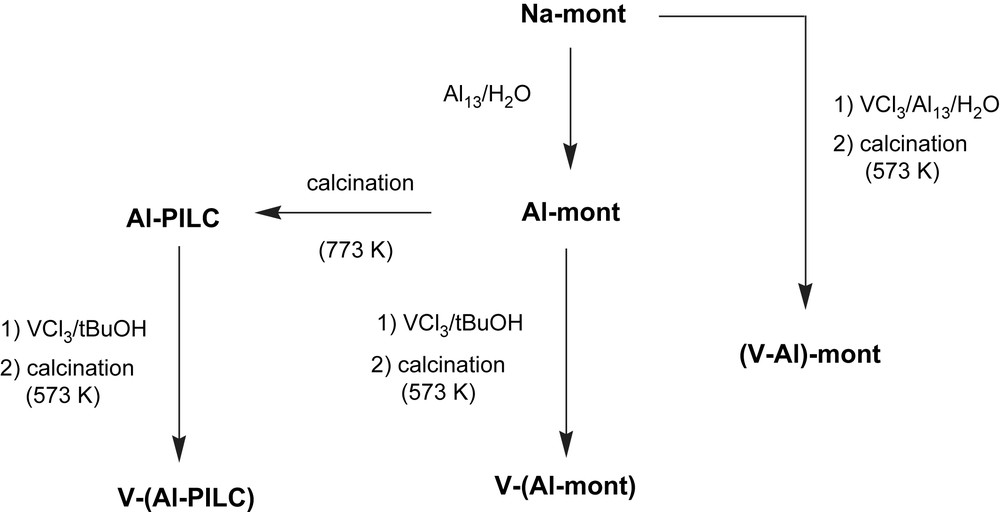
Scheme of preparation procedures leading to V-doped alumina-pillared montmorillonites.
Chemical analysis was carried out by using atomic absorption spectrometric technique on a Perkin–Elmer 3100 apparatus, after sample dissolution through acid attack. XRD patterns were recorded on a Philips PW 1130/00/60 diffractometer using Ni-filtered Cu Kα radiation and a Siemens goniometer. BET surface areas and pore volumes of the samples were measured on a Micrometrics ASAP 2000 apparatus. ESR spectra were recorded with a Bruker ER 200tt spectrometer at 77 K. 27Al MAS NMR spectra were recorded on a Bruker MSL 400 spectrometer and solution 27Al NMR spectra were taken on an AC 300MHZ.
Catalytic tests: trans-2-hexen-1-ol (0.7 g, 7 mmol) was added to a stirred suspension of the catalyst (50 mg) in a solution of tert-butylhydroperoxide (4 mmol, azeotropically dried 3 M solution in dichloromethane) in dry toluene (20 ml) under an inert atmosphere. The reaction mixture was stirred at 338 K and periodically monitored by gas chromatography (IGC 120FL).
3 Results and discussion
(V–Al)-mont samples were prepared by co-pillaring, consisting of the addition of VCl3 to the aqueous aluminic solution (Al13) with a V/Al ratio = 0.05. 27Al NMR spectrum of the pillaring solution (Fig. 2) shows a signal at 63 ppm, characteristic of the tetrahedral aluminium in the Al13 polycation, and a signal at 0 ppm, characteristic of the octahedral aluminium sites [8] both in Al13 and in other monomeric and polymeric species. The increase in the V/Al ratio reduces the intensity of the signal for tetrahedral Al and leads even to its disappearance with high ratios. This is due to the Al(OH)3 precipitation and the consequent decrease in the proportion of Keggin-type polymers (Al13).

27Al NMR spectrum of the pillaring Al13 solution.
N2 adsorption–desorption isotherms of all samples were measured. A typical isotherm is shown in Fig. 3. The adsorption isotherm is type II in the classification of Brunauer, Deming, Deming and Teller [9], whereas the hysteresis loop is of type B in the classification of de Boer [10]. Among the materials showing this type of hysteresis are those in which the pore structure is built-up of parallel plates.

Nitrogen adsorption–desorption isotherm of (V–Al)-mont sample.
The physicochemical characterization values are tabulated in Table 1. All pillared materials show an expanded basal spacing with respect to the starting Na-mont sample. Their specific areas are significantly larger indicating that the pillaring procedure was successful and the interlamellar space became accessible. The major contribution to the specific surface areas of pillared samples comes from the micropores. V-doped solids show surface areas lower than that of Al-PILC sample, due to a significant reduction in the microporosity when vanadium is incorporated. However, the co-pillaring method allows the introduction of a significantly higher amount of vanadium but the decrease in the surface area is less pronounced than in V–(Al-mont) and V–(Al-PILC) sample cases. Based on the results summarized in Table 1 (columns 2 and 5), this effect can be explained by the higher microporous volume (0.164 cm3/g) and the interlamellar space (d001 = 18.4 Å).
Physicochemical characterization of V-doped alumina-pillared montmorillonites.a
| Sample | d001 (Å) | SBET (m2/g) | <D> (Å) | Vmicro (cm3/g) | V(%w/w) |
| Na-mont | 12.6 | 41 | – | 0.005 | – |
| Al-PILC | 16.7 | 232 | 6.9 | 0.191 | – |
| V–(Al-mont) | 17.2 | 136 | 7.4 | 0.157 | 0.50 |
| V–(Al-PILC) | 17.7 | 148 | 7.9 | 0.157 | 0.37 |
| (V–Al)-mont | 18.4 | 181 | 8.6 | 0.164 | 1.07 |
a d001 = basal spacing, SBET = surface area, Vmicro = microporous volume, <D> = average dimension of alumina pillars (<D> = d001 − 9.8, where 9.8 is the clay layer thickness), V(%w/w) = vanadium content.
The XRD patterns (Fig. 4) show the presence of new diffraction peaks in the range of 19–23° (4.5–4 Å), probably due to the formation of vanadium oxide clusters, already observed for V-mont [1,2]. This effect is more evident in the solids prepared by the post-synthesis method, whereas its presence in the solid prepared by co-pillaring is only residual. This result is in agreement with a better incorporation of vanadium into the pillars through the co-pillaring method, also shown by the larger average diameter of the pillars (8.6 Å) calculated from the basal spacing and the layer thickness. In the samples prepared by the post-synthesis method, it is apparent as a shoulder corresponding to the basal spacing of montmorillonite (9.8 Å) that may originate by the expulsion of Al oligomers from the interlayer space and their replacement by the larger V–Al species. A similar phenomenon has been observed for V–Zr-montmorillonite [11]. It can be concluded that the interaction of vanadium with the pillars is greatly enhanced by the co-pillaring preparation method, as shown by the larger pillar size and the lower amount of vanadium oxide clusters.
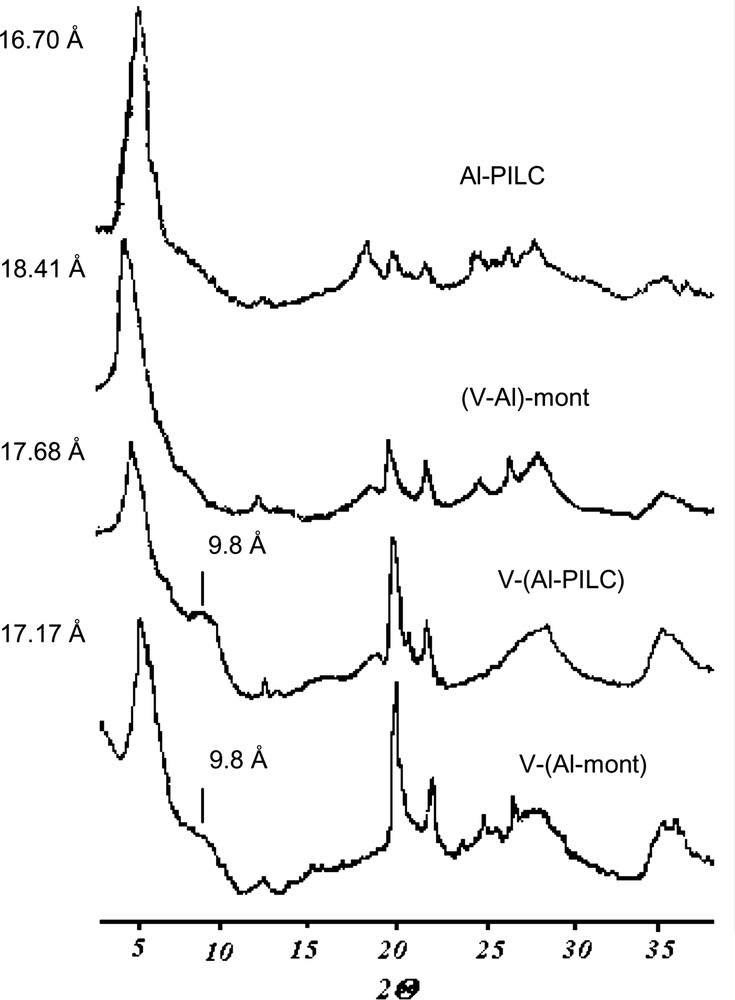
XRD patterns of V-doped alumina-pillared montmorillonites.
In order to gain some insight into the location and the nature of the vanadium centres, ESR investigations have been undertaken. All vanadium-containing samples show the typical signal of V(IV) 3d1 centres with eight hyperfine line patterns due to the interaction of the unpaired electron with the 51V nucleus (I = 7/2). The relative magnitude of the g and A components of a V(IV) centre depends on the symmetry of the paramagnetic complex and on the nature of the surrounding ligands [12–14]. Fig. 5 shows the signal of the V–(Al-mont) sample recorded at 77 K, as an example, and the parameters for all the catalysts are tabulated in Table 2. The spectra are very similar to those obtained by Rigutto and van Bekkum [15], showing a monomeric vanadyl(IV) species, with g values and hyperfine coupling constants characteristic of a distorted octahedral coordination structure with approximate axial symmetry.

ESR spectrum of V–(Al-mont) dried at 383 K.
ESR parameters of V-doped alumina-pillared montmorillonites.
| Sample | g// | A// | g⊥ | A⊥ |
| V-mont | 1.936 | 198.6 | 1.999 | 75.7 |
| V–(Al-mont) | 1.936 | 197.4 | 1.997 | 75.6 |
| V–(Al-PILC) | 1.937 | 197.5 | 1.997 | 75.9 |
| (V–Al)-mont | 1.932 | 195.6 | 1.998 | 79.9 |
The spectra have a well-resolved hyperfine structure but the baseline is not horizontal, which indicates the presence of another paramagnetic vanadium species. The superimposed broad singlet (marked with a dashed line in Fig. 5) is probably due to a strong interaction between the vanadium atoms, likely dimeric or polymeric vanadyl units [16].
The spectra recorded at 77 K and 298 K are identical, with a lower intensity of signal at 298 K. As the spectrum of tetrahedral V4+ exhibits different parameters (g// = 1.831, g⊥ = 1.980 and A// = 191 G, A⊥ = 35 G in ThGeO4 [17]), and it is detected only at 77 K [18], the tetrahedral symmetry of the V4+ environment may be discarded in our samples.
As far as the location of vanadyl centres in the interlamellar space of pillared clay is concerned, the possible sites are either close to the silica sheet of the layer or in association with a pillar. To facilitate the interpretation of the data, the calcined vanadium exchanged non-pillared montmorillonite has been used as a reference for V centres in contact only with the silicate layers (VSi centres). The analysis of ESR parameters shows that in the case of samples prepared by post-treatment, both before and after calcination, V centres may be identified mainly as species, VSi, bound to the interlayer surface. On the other hand, the spectrum of the co-pillared (V–Al)-mont sample presents parameters different from those of VSi, showing that vanadium is probably located mostly on pillars. This interpretation is supported by the effect of calcination on the samples. In fact, the V centres in co-pillared (V–Al)-mont sample are not affected by calcination in air which shows its poor accessibility related to its higher stability bound to the pillars. This is not the case for pillared samples doped by exchange, where there is a much larger decrease in signal intensity in ESR after calcinations due to the oxidation of vanadium from V(+IV) to V(+V).
Some more information on the location of the vanadium species can be obtained from the 27Al MAS NMR spectra of alumina-pillared montmorillonites. The spectrum of Al-PILC shows a dissymmetrical peak at about 2 ppm (Fig. 6), which corresponds to the superposition of the signals of octahedral aluminium of the layers and the polycations Al13. Another weak broad band corresponding to tetrahedral aluminium is also present at about 60 ppm. After introduction of vanadium the band corresponding to the octahedral Al suffers a remarkable widening in the case of (V–Al)-mont. Such a widening can be explained by the strong interaction of the vanadium species with the alumina pillars, as it was already deduced from the study by ESR. This widening is less important in V–(Al-mont) sample, probably due to a random distribution of the vanadium centres. Finally this band in V–(Al-PILC) shows the same width as that in Al-PILC, demonstrating the same nature of pillars in both cases. A similar trend is observed in the case of the band corresponding to the tetrahedral Al. The shift of this band is more important in the case of (V–Al)-mont, showing a modification of the pillars by the inclusion of vanadium.

27Al MAS NMR spectra of pillared clays: (a) Al-PILC; (b) V–(Al-PILC); (c) V–(Al-mont); (d) (V–Al)-mont; (e) montmorillonite.
Thus, on the basis of the physicochemical characterization, two structural models of V-doped Al-pillared clays might be proposed (Fig. 7). In the case of solids prepared by post-treatment, the density of pillars is lower, and the vanadium centres are distributed on both the clay surface and the pillars. The sample prepared by co-pillaring presents a more uniform distribution of pillars, with vanadium mainly situated on them.

Schematic models of V-doped alumina-pillared montmorillonites.
The catalytic activity of the three V-doped solids was tested and compared with the catalytic activity of two other vanadium catalysts such as V-mont and V2O5/Al2O3 in the epoxidation of an allylic alcohol (trans-2-hexen-1-ol) with an alkyl hydroperoxide. The results are tabulated in Table 3.
The catalytic activity of V-doped alumina-pillared montmorillonite and other vanadium catalysts.
| Catalyst | V (%w/w) | SBET (m2/g) | Yield of epoxideb | TOF epoxidationc | TONd |
| V2O5/Al2O3a | 7.5 | 112 | 35.5 | 11.2 | 19.3 |
| V-mont | 6 | 18 | 30.75 | 13.5 | 21 |
| V–(Al-mont) | 0.50 | 136 | 34.50 | 117.3 | 281 |
| V–(Al-PILC) | 0.36 | 148 | 25.37 | 48 | 287 |
| (V–Al)-mont | 1.07 | 181 | 23.27 | 16 | 88 |
a Synthesised following the method described by Lapina et al. [19] using an aqueous solution of NH4VO3 (5 mmol of NH4VO3 added to 1.0 g of γ-Al2O3).
b Referred to the maximum for overall conversion of TBHP, after 5 h.
c Millimole of epoxide (mmol V)−1 h−1 from data after 30 min.
d Millimole of epoxide (mmol V)−1.
As can be seen the catalytic performance of V-doped alumina-pillared montmorillonite varies with the amount of introduced vanadium and the method of its insertion. V–(Al-mont) is the more efficient catalyst, both in yielding epoxide and mainly in TOF epoxidation parameter.
However, the textural and structural studies and the location of vanadium species show that V–(Al-mont) and V–(Al-PILC) exhibit the same properties. This indicates that the difference in the catalytic activity of both the solids is due to the amount of vanadium.
The co-pillaring method allows the introduction of a significantly higher amount of vanadium but this does not improve the catalytic activity. Two hypothesis could be presented in order to explain these results. V centres are located essentially on pillars, thus the active phase is present essentially in the micropores, where the diffusion limitations may play a role on the lower catalytic activity. Secondly, according to ESR study, the vanadium species have poor redox properties and exist mainly in low oxidation state (+IV). Indeed, we have been shown [2] that vanadium species, in higher oxidation state (+V), are found to be the catalytic centre in epoxidation of allylic alcohols.
The possibility of the homogenous catalysis by the vanadium ions leaching from the three vanadium-containing solids has strictly been investigated. First the filtrate after the first reaction did not show any catalytic activity for the second oxidation reaction. Secondly, the vanadium content in the filtrate was analysed by atomic adsorption spectroscopy and was found to be below the limit of detection.
In order to evaluate more the catalytic performance of the three V-doped alumina-pillared montmorillonite, two other vanadium catalysts, such as V-mont in which V centres are in contact only with the silicate layers [2] and V2O5/Al2O3 synthesised by the impregnation method using an aqueous solution of NH4VO3 as Vanadium-source, have been studied in the epoxidation of trans-2-hexen-1-ol (Table 3). Compared to the V-doped alumina-pillared catalysts, a higher amount of vanadium was observed both in V2O5/Al2O3 and in V-mont catalysts but this does not noticeably improve the yield of epoxide and the TON and the TOF epoxidation parameters are much lower. Therefore, V-doped alumina-pillared montmorillonite catalysts are more efficient and this is probably due to the development of higher surface area which is the origin of a well-dispersed state of active vanadium species.
4 Conclusion
The preparation procedure of V-doped alumina-pillared catalysts conditions the structural and textural properties, as well as the type of V centres of the final material. All the samples are predominantly microporous, whose volume is very sensitive to the preparation method. In the case of V–(Al-PILC) and V–(Al-mont) samples, it becomes reduced, due to the partial collapse of the pillared structure. The effect is assigned to the sagging of the layers after expulsion of some of pillaring Al-oligocations by vanadium species.
According to ESR, only in the co-pillared material the V centres are associated exclusively with the pillars, whereas in the two other samples vanadium species are located both on the pillars and on the silicate layer.
Textural and compositional differences affect the catalytic performance of the samples. The sample obtained by co-pillaring method is the least active of all samples. In fact, the presence of vanadium species associated mainly with the pillars shows poor redox properties leading to a low catalytic activity which is not the case for the vanadium bounded to the interlayer surface which is more accessible. Compared to other vanadium catalysts, the three V-doped alumina-pillared catalysts are more efficient for the epoxidation of trans-2-hexen-1-ol and this is probably due to the well-dispersed state of active vanadium species.


