Preface
Undoubtedly, Prof. Jacques Livage has been one of the main driving forces behind sol-gel chemistry as well as one of the most impressive workers in this field of Chimie douce routes to advanced materials [1–38]. His works have guided many researchers to use this chemical synthesis method to obtain new materials or previously known materials with novel and enhanced features. He was also a pioneer in noticing that the barriers between the fields of chemistry, biology and physics had to be lifted at once, because the advancement in knowledge required the combined efforts of these disciplines. Nowadays, no one doubts that science needs a multidisciplinary research, in which the so-called transversal supply of knowledge from and between the domains of chemistry, biology, materials science and medicine will empower the know-how and applications that shall, undoubtedly, give rise to new advances in science and technology.
In this special issue devoted to Prof. Livage, I would like to pay homage by reviewing a type of materials, the bioceramics, selecting those among them which have been synthesised by sol-gel route within our research group.
This is my tribute to you, Jacques!!!!
1 Introduction
The field of biomaterials requires the input of knowledge from very different areas so that the implanted material in a living body performs adequately. The biomaterials discipline is founded in the knowledge of materials science and biological clinical science, with the final aim of achieving a correct biological interaction between the material and the host. In this sense, biomaterials are an excellent example of a pluridisciplinar field where the material, developed by materials scientists and engineers, has to be validated and must perform its task inside the human body, under the expertise of physicians and biologists; the final outcome must be analyzed and coordinated by all the intervening scientists. The process starts when a specific need is identified, then the idea of a potential implant is developed, to conclude with the final insertion of the implant in a patient. The whole process is very long because several stages have to be verified: material synthesis, design and manufacturing of the prosthesis, combined with multiple material tests. Besides, it must also pass all regulatory requirements before its application to patients.
In order to follow the path defined by the evolution of bioceramics within the field of biomaterials, some statistical data should be firstly reviewed. At the end of the 20th century, the life expectancy reached values around 80 years. Those values are spectacular if compared with those corresponding to early 20th century, around 40 years, while in Imperial Rome the life expectancy was only 22 years of age. Therefore, 19 centuries passed before the expected lifespan doubled and then, in just one century, the 20th century, it doubled again. This spectacular rise is still ongoing, increasing the demand and the need for biomaterials. The ageing of the population involves certain problems that were not so generic some time ago, since fewer people reached those ages where the incidence of such diseases is more evident. Osteoporosis is a fine example; this disease attacks the bone as a consequence of a major lack of osseous mass. Science and technique are looking for solutions to the problems derived from an ageing population. The use of biomaterials can solve some of these issues.
Biomaterials could be defined as “implantable materials that perform their function in contact with living tissues”. Biomaterials and tissue engineering sciences aim to develop materials which can be implanted in the human body to replace damaged tissues. Depending on the function to perform, they can be manufactured from very different materials. It is known that the reactivity of solids begins on their surface. This general statement is of particular importance in the field of biomaterials, since they will be in contact with an aqueous medium and in presence of cells and proteins. Nowadays, it is possible to manufacture implants for any part of our body, except for the brain. Obviously, different types of materials are in use depending on the tissue to be replaced. Regarding the materials to be used, it is critical to bear in mind that a group of biomaterials will be applied in body reconstruction functions, hence they must perform their duty for an undefined period of time, that is for the rest of the patient's life. Besides, another group of biomaterials will be used in temporary body support functions. This “permanent” or “temporary” feature allows for a larger and better choice of materials for implant manufacture.
If we focus on functional artificial biomaterials, the choice has to be made among metals, polymers and ceramics. Each group exhibits some a priori advantages and drawbacks. Ceramics, for instance, are the most biocompatible materials and can be obtained with biostable, bioactive or bioresorbable properties, but their main drawbacks are their hardness and fragility. Metals exhibit problems of corrosion and toxicity, but their mechanical behavior is optimum. Polymers offer many possibilities depending on their chemical composition and structure (biodegradability degree, hydrophilic/hydrophobic ratio, toughness/flexibility, etc.), but very few have shown good bioactive properties (for instance, Poliactive®) to ensure the implant osteointegration. Therefore, it is important to reach the best compromise possible, and it is quite usual to use the three types of materials in the same implant. This is the case of a total hip joint prosthesis which presents a metal beam, partially coated with a bioactive ceramic, while the head is made of an inert ceramic and the socket is made of polymer.
2 Biological apatites
Turning back now to bioceramics, and before dealing with their production in the laboratory, it should be recalled that the inorganic phase of our bones is apatite. Apatite is the term of a very abundant mineral in the earth's crust, with Ca10(PO4)6(OH)2 as general formula [39]. Its structure has the special ability to accommodate several different ions in its three sublattices [40,41]. Bone apatites can be considered as basic calcium phosphates. Biological apatites are formed in living bodies through a biomineralisation process where cells and proteins are involved. The formation of hard tissues starts with an amorphous calcium phosphate which evolves towards a nanocrystalline apatite, calcium-deficient, always with presence of carbonate ions. It is, therefore, a non-stoichiometric nanoapatite. Both bones and shells of molluscs are composite materials formed by a matrix that constitutes their organic component and an inorganic salt that plays the role of inorganic component. In the case of bones, this inorganic salt is a calcium-deficient carbonated nanoapatite. The size of these apatites is nanometric, ranging from 25 to 50 nm. They grow at the mineralization sites of the collagen molecules. These molecules are grouped together forming collagen fibers. This is a very broad explanation of the structure of our bones [41]. The hard tissues of vertebrate animals are bones. X-ray diffraction confirms that the apatite in bones and dentine exhibit particle sizes in the order of nanometers. The apatite particles in the enamel are somewhat larger, with oriented nanocrystals that reinforce the mechanic properties, in order to succeed in the protection and mastication functions of this material. Fig. 1 shows its arrangement, leaving voids in the micron range. Therefore, our bones are formed by nanometric apatites arranged in a hierarchical structure with porosity of a micron scale, so that cells can perform their bone formation and regeneration tasks. The orders of magnitude of biological apatites and cells are very different, since it is in the nanometer range for the former and in the micron range for the latter. Bone porosity is necessary for several physiological functions performed by the bone [42,43]. Fig. 1 depicts the hierarchical structure of bone and its complexity, symbolising from the skeleton to the collagen molecules where, at certain locations, biological nanoapatites grow. Apatite mineral crystallises at the Earth's crust, with a high degree of crystallinity as evidenced by X-ray diffraction. Biological apatites grow in living species, with poor crystallinity and nanometer size [44].
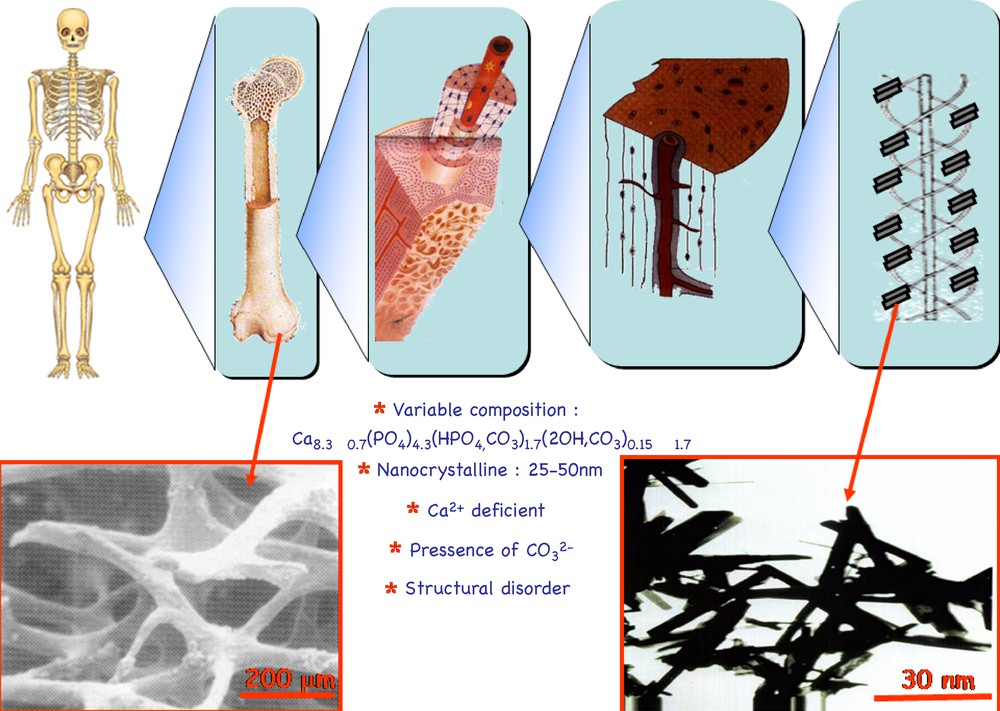
Hierarchical arrangement of bone tissue. The picture on the lower left side shows the porosity of bone tissue, in the micron range. Inset picture on the lower right side shows nanometer-sized apatite crystals.
Bearing in mind the natural bone model, an approach to the fabrication of bone would be to combine the organic and inorganic phases to achieve a nanoceramic with a certain viscoelasticity, allowing for cell activity and, obviously, being biocompatible.
3 Artificial bioceramics
In order to obtain nanoapatites out of the biological environment, it is necessary to use wet route synthesis methods. Sol-gel method is a good option. This method allows one to obtain both nanoapatites and glasses. These glasses can be used as precursors of apatites as will be discussed later.
There are many other wet route methods available to obtain nanometer-sized calcium phosphates [45]. Therefore, chemistry offers many options to obtain, at the laboratory, apatites with similar composition and size to those obtained from living species. However, before describing them in detail, it is worth mentioning which ceramics have been used in clinical practice and how has evolved the knowledge on these materials in the race to achieve better performances. We may begin by reviewing the present solutions for bone repairing, those which are being applied today.
Not very long ago, the most popular solutions involved the use of natural materials, using bone from the patient himself, from a bone donor bank or from animals. But there are disadvantages in this method: in the first case, the patient has to endure two surgical interventions instead of one, and there are general risks of infection (HIV, Creuzfeld-Jacob…) in all the others. This is why the artificial materials are gradually being considered with more interest. Among them, apatite is placed in a very important position [46].
When searching for ceramic bone substitutes, the chronology has been as follows: it all started in the 1950s, and the first aim was to use inert materials which had no reaction with living tissues. Later on, in the 1980s, the trend changed towards exactly the opposite; the idea is to implant ceramics that react with the environment and produce newly formed bone. And then, in the current century, we are searching for new ceramics [47]. We may now analyse this situation.
4 Ceramics for hard tissue replacement
The first generation is formed by inert ceramics. From the chemical point of view, two well-known examples are zirconia and alumina. They are primarily used in the fabrication of femoral heads [48]. But these ceramics, in a similar way to what happens with metallic and polymeric biomaterials, experiment foreign body reactions. Therefore, and although they are biocompatible, the body will react against them due to their foreign nature; the implant is then surrounded by an acellular collagen capsule which isolates it from the body [49]. In this way, the material will never transform itself into bone, and its artificial nature prevails.
5 Ceramics for hard tissue regeneration
The search for bioactive ceramics yielded promising results in the 1980s. These ceramics can react with the physiological fluids forming biological-type apatite as a by-product of said reaction; in the presence of living cells, this apatite can form new bone. Among these ceramics, we can mention calcium phosphates, and several compositions of glasses and ceramic glasses. For medical applications, these materials are provided in the following formats: powder, porous pieces, dense pieces, injectable mixtures and coatings. They have excellent features in terms of biocompatibility and bioactivity, but their mechanical properties are very poor.
5.1 Bioactive glasses
Larry Hench proposed in 1968 an imaginative route to obtain new bone from a glass [50]. A glass is a solid matter without crystal structure, a disordered solid which is therefore highly reactive [51].
In order to study how these glasses react inside the body, a very simple first assay would be to introduce a piece of glass in an aqueous solution and to analyze the evolution process both in the glass and in the solution. This solution can be prepared with the same ions present in the human plasma. In this way, we shall be working a little closer to in vivo conditions. And in this in vitro study, it is possible to monitor the ion exchange between glass and solution, the evolution of the pH value of the solution, the changes in the glass surface where [52], if it is really bioactive, a newly formed apatite coating should be formed. If the evolution of the glass surface is monitored by X-ray diffraction, the starting point will be the typical diffraction scan of an amorphous material, as usual for a glass. But, upon a few days (commonly between 1 to 7 days depending on the glass composition) of contact with the fluid, we can see the formation of some broad and poorly defined maxima [41,52,53]. If we compare this result with the X-ray diffraction scan of a natural bone, we can see how similar they are [54]. Simultaneously, infrared spectroscopy indicates the formation of phosphate and calcium bands [55]. This result confirms the evolution from the amorphous glass to the formation of a carbonated and poorly crystallised apatite. Scanning electron microscopy (SEM) also helps to visualise what is going on (Fig. 2). In this example, the starting material was a glass of composition 80% SiO2, 17% CaO and 3% P2O5. It has been immersed in the fluid for 7 days and after that period, the study was performed again. A layer of spherical particles was then formed onto the glass surface. And the chemical analysis indicated that this layer was only formed by calcium and phosphorus. A higher magnification revealed that each sphere was formed by crystal aggregates. Transmission electron microscopy (TEM) performed on these aggregates also revealed that they were formed by thousands of nanocrystallites, with sizes in the order of 50 nm, with a Ca/P ratio of around 1.20 [56], that is, similar to biological apatites which are always Ca-deficient and therefore, with Ca/P ratios below 1.67 (ratio depending on the type of bone, location and age). Electron diffraction allowed one to identify a possible apatite phase. Therefore, the reaction product of the glass and the fluid was an apatite similar to biological ones. If these observations are evolved from micro to nanoscales, images of nanometric-sized apatites formed can be obtained, with needle like arrangements. Although their bioactivity is excellent, the great problem of glasses is that their mechanical properties are very poor, rendering it impossible to use them in the repair of large osseous defects. However, these glasses have an excellent field of application in the filling of small defects, where the rate of regeneration is the main concern, and where mechanical properties are just a secondary aspect.
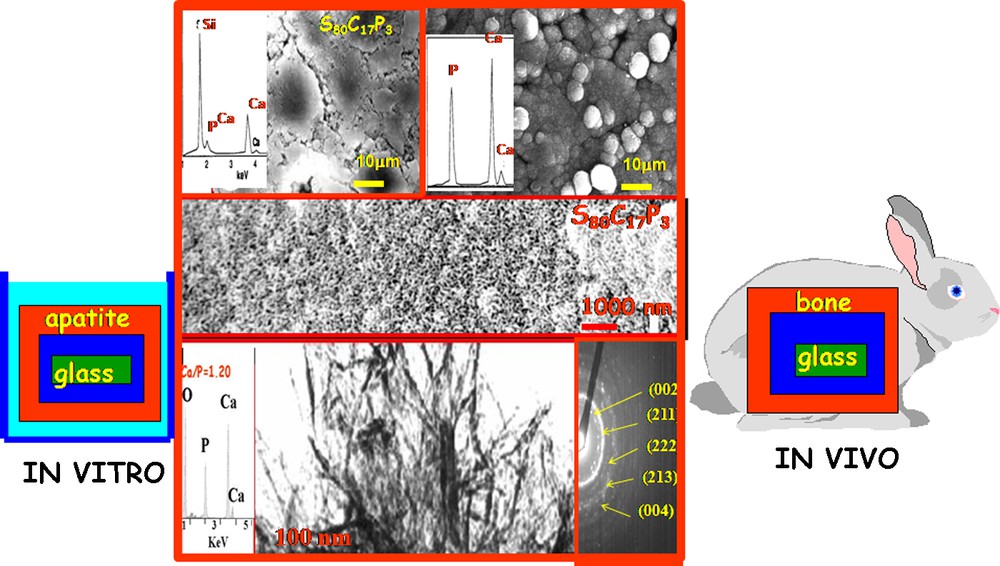
Scanning electron microscopy and transmission electron microscopy (TEM) images confirming apatite formation onto the glass surface upon in vitro assay. When in presence of cells and proteins, newly formed bone tissue is obtained.
In vivo assay with these glasses, implanted in the femur of rabbits, allowed one to confirm that the bone regenerates simultaneously to the degradation of the glass piece [57]. However, the glass was not completely transformed. The glass was implanted in the form of a dense piece and the surface reaction did not reach its inner region. This evidence is a question to be considered; therefore, it could be interesting to prepare glass implants in the form of porous pieces. If it works correctly, the kinetics of the bioactive process would clearly increase. And also, since the fluid would reach the inner positions more easily, the transformation of the whole glass would also improve. But it is also obvious that the mechanical properties would be even worse, so we must focus again on those applications where they are not needed. For instance, periodontal surgeries with regenerative purposes that are often needed before metallic dental implantations. It is therefore important to choose wisely the possible materials to use, depending on the required application. It is a very wide field that needs to be explored and worked.
5.2 Bioactive glass ceramics
Glasses also can be used as precursors in the production of glass ceramics [58,59]. In fact, a certain heat treatment to a glass yields a glass ceramic, which exhibits better mechanical properties, among other advantages. For the moment, the porosity will not be considered and the discussion will remain focused on dense materials. If the bioactivity study is now performed with glass ceramics, considering for instance a sample of composition: 70% SiO2, 26% CaO and 4% P2O5, it can be observed that since it is formed by different phases, some more soluble than others, the bioactivity process starts at the more soluble areas [60]. The specific microstructure of glass ceramics reinforces the mechanical properties of the whole piece. Therefore, it is possible to obtain bioactive glass ceramics with mechanical properties much closer now to those of the natural bone [61–63].
5.2.1 Organic–inorganic hybrids
The organic–inorganic hybrids can also be considered among the new ceramics for clinical applications [64–68]. These materials have been developed on the basis of SiO2, CaO, TiO2, etc. mixed oxide systems as inorganic phases, associated to siloxanes derived ormosils, acrylic polymers, caprolactones, etc. It is important to ensure their biocompatibility and, if possible, bioactivity. If this aspect is verified, the aim then is to obtain adequate mechanical properties for applications dealing with bone replacement. It is a huge field of research.
Another field of discussion is the possibility to obtain hybrid materials with adequate mechanical properties for bone replacement applications, and also with the bioactivity of the glasses. In fact, it is possible to synthesise this type of hybrids with mechanical features similar to those of natural bone [69]. It is feasible to obtain monoliths with moulded shape and size, and with bioactive features. Therefore, still from the point of view of second generation bioceramics, it is possible to improve their mechanic properties. However, the discussion is still focused on dense materials.
5.2.2 Magnetic glasses and glass ceramics mixtures
Using magnetic bioactive glasses and glass ceramics, it is possible to design magnetic materials that fulfil two roles simultaneously: to regenerate the bone thanks to their bioactivity and to treat cancer in bone tissues, through hyperthermia treatment of osseous tumours. This treatment consists in heating tumors up to temperatures between 43 and 47 °C. Within this interval, the malignant cells are selectively destroyed whereas the healthy ones only undergo small and/or reversible damage [70–72]. Among the different magnetic phases included in these materials, magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the most commonly used.
5.2.3 Calcium phosphate cements
Another type of materials in this second generation of materials is the group of calcium phosphate cements. The idea is to inject the material in the osseous cavity, with a very quick response of bone attachment and simultaneous regeneration. At present, there are several patents for these types of cement. First of them all was Norian SRS, based on the work of Constantz, published in Science in 1995 [73].
There are already several and very diverse applications for these cements. Some of them are already at a commercial stage and in use but, as it has been already mentioned about the glasses, they are only having good results in the repair of small osseous defects. Research is under way to add a certain degree of porosity to these cements so that their transformation into bone is as complete as possible [74,75]. However, when placed under heavy loads, their performance is still not too good enough.
These bone cements could be used to produce three-dimensional scaffolds hosting osteoblast cells, although this is a field yet to be fully controlled; the main drawback is the high bioactivity of these cements, which react too rapidly with the environment. When in contact with the culture medium, these cements absorb calcium from it, releasing phosphates and lowering the pH values. This is hopefully a matter which can be controlled and solved in the near future.
5.2.4 Ordered mesoporous silica materials
The discovery of highly ordered mesoporous silica [76] was quickly recognized as a milestone that could lead to a variety of important applications in host–guest systems [77]. Silica-based mesoporous materials have unique structural characteristics, since that an amorphous silica network constitutes the wall of well-ordered arrangement of pore system and cavities [78].
The synthesis is based on using surfactants that act as templates. The larger the micelle is, the larger the pore to be obtained. Other mesoporous materials can be synthesized by other methods, but they will not be dealt with in this review.
At this moment, the field of chemistry offers many possibilities to synthesise silica mesoporous materials but, whichever route is chosen, the outcome should be an ordered porous silica structure. In other words, using a very similar chemical composition to that of bioactive glasses, it is possible to obtain mesoporous structures. Predictably, such structures should have bioactive behaviour when in contact with physiological fluids, in a similar fashion to what was previously described in glasses (Fig. 3). If a parallel study is performed, similar to what was done with glasses, and if the mesoporous material is bioactive, another ceramic able to regenerate the bone tissue may have been obtained. And, in fact, this is the case [79,80].
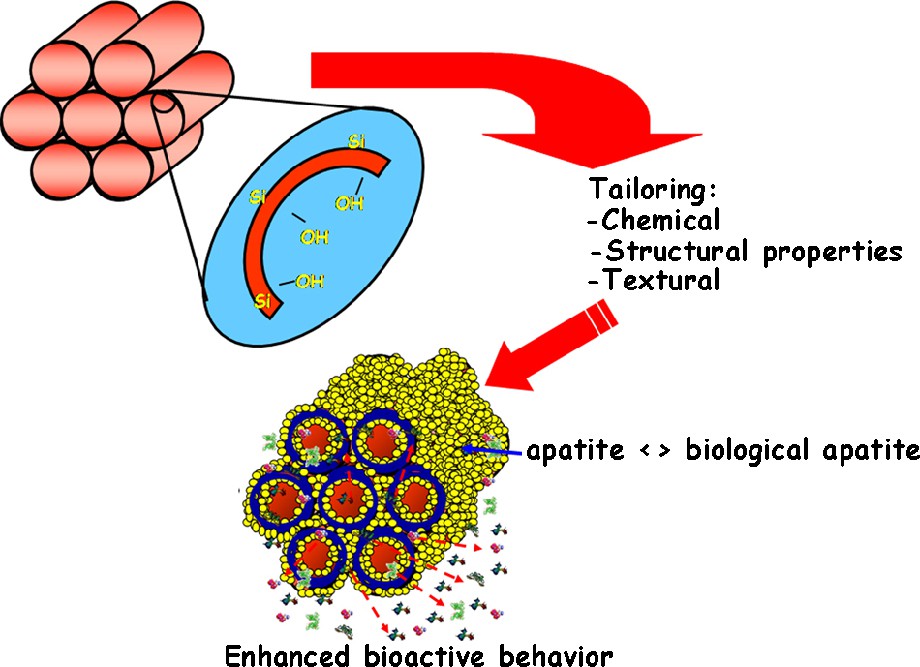
Silanol group on the surface of ordered silica mesoporous materials which, when in contact with physiological fluids, enable the apatite formation.
This type of ordered mesoporous materials can behave as bioactive glasses, but also including a pore channel system with dimensions that allow the inclusion of different molecules with biological activity. For the moment, we should just focus on the following idea: ordered silica mesoporous materials are able to form biological type apatites, when in contact with physiological fluids [81,82].
These silica mesoporous materials allow one to load biologically active molecules such as drugs, peptides, proteins, or growth factors. Hence they play a double function: bioactivity and release systems for biologically active molecules which may help and improve bone regeneration [83–86]. Different parameters are governing the loading capacity of biologically active molecules [87–89] (Fig. 4). Among them, we can mention the pore diameter as size selective parameter, and the surface area and functionalization as factors that improve the adsorption capacity. A large range of molecules from small drugs to proteins can be confined inside the pore network of such materials [90,91].
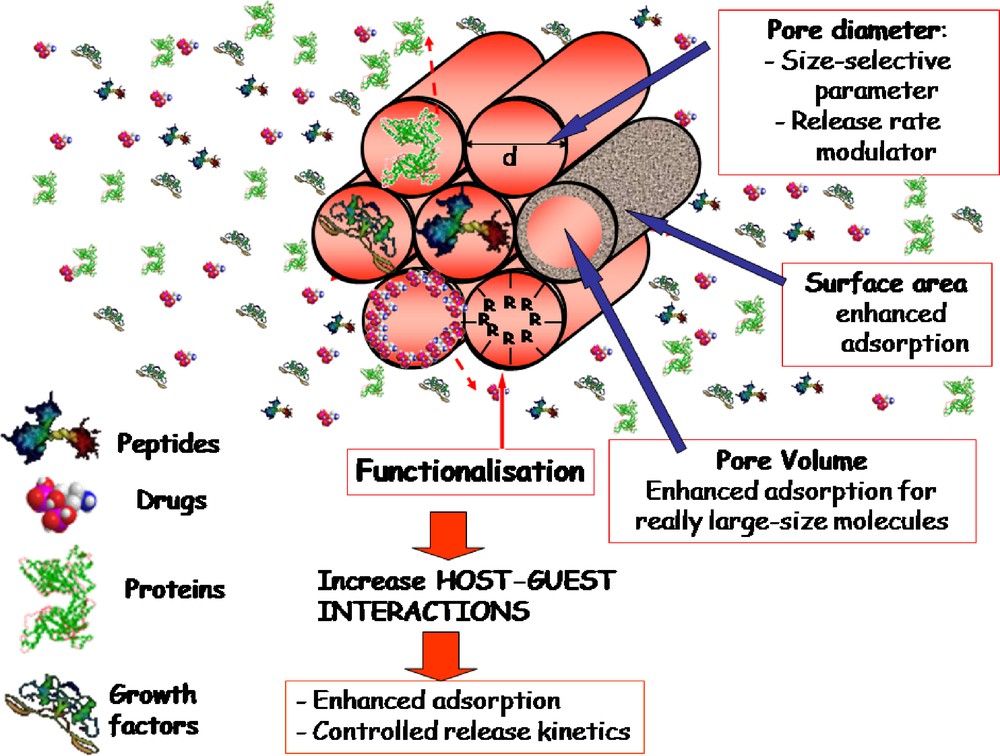
Parameters that govern the loading capacity of biologically active molecules.
5.3 Templated glasses
Another interesting attempt is to use the synthesis methods of silica ordered mesoporous materials but with the same compositions of glasses. In this way, mesoporous materials with the excellent bioactivity of glasses could be obtained which, according to some studies, exhibit the highest kinetics of apatite formation [92]. In fact, the use of surfactants in the synthesis of equivalent compositions to those of bioactive glasses allows to obtain “ordered mesoporous templated glasses”. A comparative study between traditional sol-gel glasses and these so-called templated glasses allows one to show several differences.
Its specific surface is roughly double. Therefore, equivalent materials (in terms of Si content) have been obtained, but with a much higher contact surface for the physiological fluid; this fact allows one to predict that the apatite formation kinetics shall be much higher, and also with an ordered mesostructure.
The transmission electron microscopy study, also in the electron diffraction diagrams, with three orientations, allows one to calculate the space group of this new phase [93]. Also the bioactivity study of these templated glasses reveals that all of them are bioactive and, as expected, the apatite formation is evidenced after much shorter times than in traditional sol-gel glasses. Besides, it should be recalled that they exhibit an ordered mesostructure. This fact opens new possibilities to potential applications in loading and releasing molecules, for instance.
The comparison of traditional sol-gel glasses with templated bioglasses shows that the ordered mesoporosity of the latter increases clearly the bioactivity rate, reaching after 4 hours the same results that are observed in traditional glasses after 3 days [93]. Since the bioactive process is a surface cascade of events, the release of possible included drugs can be affected by the new apatite formation. This fact must be carefully considered when designing templated bioactive glasses with drug delivery purposes. The textural and structural properties of “templated bioglasses” give rise to their biomimetic mechanism. A sequential transition has been observed, from amorphous calcium phosphate, through octacalcium phosphate, towards calcium-deficient carbonatehydroxyapatite maturation [93]. This is similar to the in vivo biomineralization process. The biomimetic bone mineralization can be followed by TEM. After 1 hour soaked into a simulated body fluid, the templated bioglass generates a large amount of newly formed amorphous calcium phosphate [93]. The templated bioglasses develop nanocrystalline oval biphasic nuclei of octacalcium phosphate with a small fraction of hydroxiapatite. The transformation from oval octacalcium phosphate nuclei to needle-shaped apatite nanocrystals is verified later [93].
6 Reconsidering first and second generation bioceramics
First and second generation ceramics have been already deeply investigated, whether as a single material or combined with other materials. At this point, perhaps it should be considered briefly what the balance between mechanical properties and bioactivity implies. In second generation ceramics, the aim was primarily to improve their bioactivity, while trying to reach mechanical properties similar to those of natural bone. As already shown, this route allows one to obtain bioactive ceramics with improved mechanical properties. It has also been found that better mechanical properties can be achieved for more dense materials, although the transformation into bone becomes less complete. As a consequence, it was necessary to study the option of porous materials. On the other hand, driven by biological requirements, it became obvious that the ceramics have to be porous; and such porosity has to exhibit a hierarchical structure. It is necessary to introduce in these materials porosity values in the range of microns so that they can fulfil physiological requirements in their use as scaffolds for tissue engineering. This is in short the path followed to reach third generation bioceramics [94] (Fig. 5).
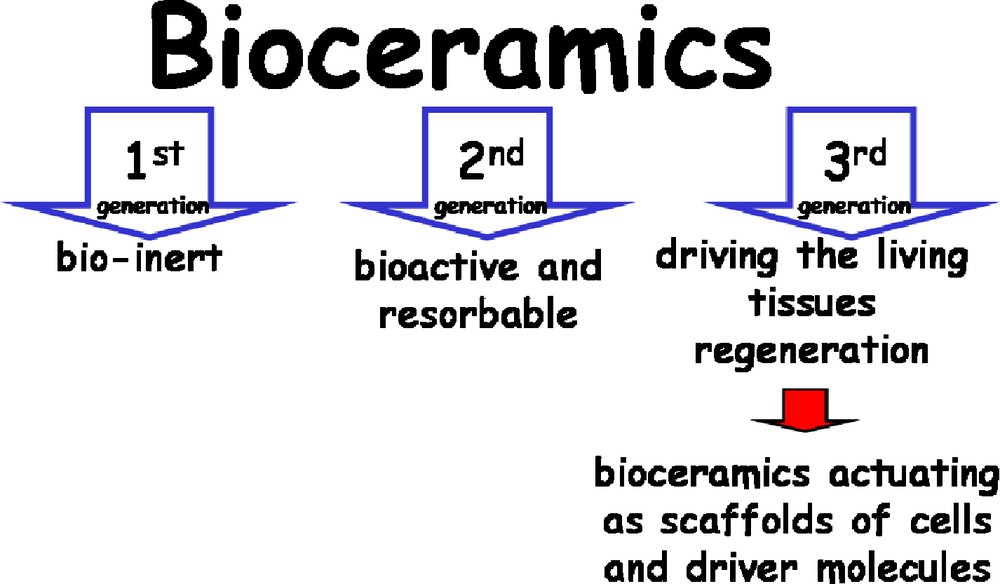
Layout of the three generations of bioceramics.
The main purpose now is to obtain porous ceramics that act as scaffolds for cells and inducting molecules, able to drive self-regeneration of tissues.
With these requirements, second generation bioceramics could still be used, although with added porosity. But new advanced ceramics can also be designed. This porosity should be in agreement with biological requirements [95]. As starting material: nanometric apatites, shaped in the form of pieces with interconnected and hierarchical porosity, within the micron range.
7 Scaffolds for tissue engineering
The design of scaffolds able to guide cell growth is an important challenge in tissue engineering. The aim is to fabricate pieces that support and structure the newly formed tissue, and said pieces have to be made of the most suitable materials for these tasks. Biological cells must be cultured onto this support and subsequently give rise to the growth of new tissue. In order to work in potential hard tissue replacement solutions, it is required to know and bear in mind the bone regeneration process. Wolf's law dictates that the bone remodels itself as a function of those forces acting on it, hence preserving its shape and density. The mechanical loads of stress, compression, flex and torsion in bones and the interstitial fluid contained in them generate stresses and deformations at the microscopical level, which in turn stimulate the cells.
The present target in biomaterials is to produce three-dimensional scaffolds with interconnected porosity so that cells can proliferate and form tissue in a similar way to the process in human tissues.
The use of finite element calculation methods contributes to the study of interactions between materials, mechanical stimulations and biological responses, since it allows one to simulate the conditions generated by bioreactors in the scaffolds and the effect and deformation at each point of the scaffold can be quantified, for instance, at each node, and then to quantify the relationship between mechanical loads and cell differentiation [96].
The fabrication of scaffolds for tissue engineering requires choosing a conformation method that yields pieces with interconnected porosity and pores in the 20 to 400 micron range [47]. The main purpose now is to obtain porous ceramics that act as scaffolds for cells and inducting molecules, able to drive self-regeneration of tissues. In this sense, the conformation methods must allow one to obtain porous scaffolds keeping the small particle size of the ceramics, in other words, methods which do not need high temperatures. At present, the aim is to find bioceramics which induce the regeneration of hard tissues stimulating the response of the cells involved. The requirements for these ceramics are to act as a scaffold and also to be porous so that the cells can do their job. This porosity implies a certain sacrifice of their mechanical properties. It is also required a certain intelligent behaviour, so that they can modify their properties in response to certain stimuli. It also it required, in some cases, to allow the loading of biologically active molecules onto such ceramics.
It is worth recalling the concept of porosity and its range of order. Those materials with mesoporosity between 2 and 50 nm are of interest for applications where drugs or biologically active molecules are loaded, and later released to help in the bone regeneration process. Macroporous materials, where the pore sizes are in the order of microns, are adequate as scaffolds for tissue engineering (Fig. 6).
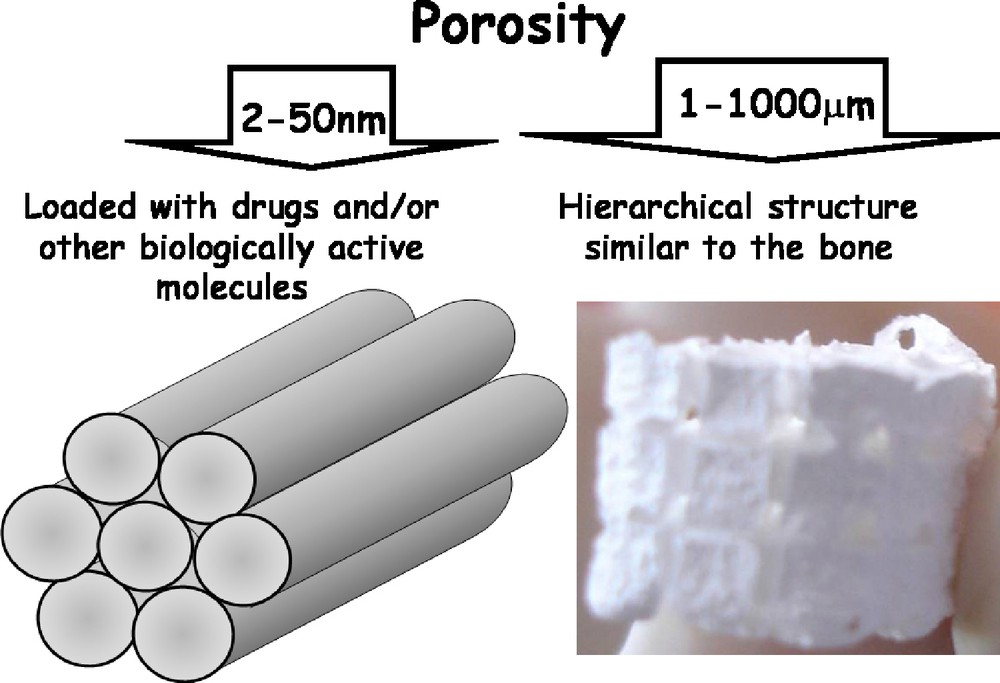
Orders of magnitude for drug delivery and tissue engineering respectively.
Therefore, the first step would be to find methods of conformation that yield pieces with interconnected porosity, with certain values of porosity and in the range of microns. And this must be possible with all the bioceramics previously discussed. One option would be to use polymer scaffolds as negative of the desired ceramic piece. After conforming the piece, the polymer is removed by an acid or basic attack, or using mild temperatures. These pieces, with designed porosity, preserve their bioactive behavior, where apatite has grown throughout all the free surfaces [97,98]. Nowadays, there are several conformation methods which allow one to obtain pieces at room temperature. Besides, working at room temperature allows one to include biomolecules of interest in many cases to treat different diseases, or to improve the treatment of various bone pathologies. It is also possible to fabricate pieces with them, with the required macroporosity for bone oxygenation and vascularisation, to be used as scaffolds for tissue engineering.
An important challenge is to design materials that can help the human body to improve its regeneration features, not only recovering the structure of the damaged tissue, but also its function [99]. Tissue engineering aims to restore the structure and function of the tissues or damaged organs. The repair starts by in vitro techniques on scaffolds cultured with cells, to be then implanted in the host [100]. Usually, this technique requires porous scaffolds that are biocompatible and bioresorbable. Tissue growth factors are incorporated into this material in order to promote the cellular function. This scaffold acts as a 3D template for the initial cell attachment, followed by the formation of the tissue.
Insofar there have been developed more sophisticated systems by controlling the implant-tissue interface, which leads to gradually discard the artificial purposes to give way and promote the natural agents instead.
8 Current trends
Tissue engineering attempts to develop artificial materials able to replace biological tissues in situations where the human body cannot perform said replacement by itself. One attempt consists on designing biomimetic materials that combine synthetic materials with cellular recognizing positions. These hybrid materials can yield surfaces with better properties. There are some difficulties to choose the specific type of cell from the huge universe of them. It is also difficult to induce functionality and architectures in multiple cells when they react with the surface of a biomimetic material. Nowadays, the research is focusing into protein modeling using ligands with high specific recognition and with spatial location only possible in certain areas to achieve a correct cellular organization. However, the microelaborated proteins into their surfaces can modify their conformation and, therefore, they can be denaturalized in this surface, which would produce a drastic decrease in the efficiency response of the cellular receptor in the surrounding of the living tissue. The challenge consists in obtaining surfaces to which proteins can attach without modifying the cells activity.
Simultaneously, an overview of the research on bioceramics along time reveals that their development and advances are, to a certain degree, related to the decrease of our expectations as materials researchers. The first generation of inert ceramics aimed at substituting natural bone; the second one was aimed just at mimicking some biomineralization-related functions; finally, the purpose with the third generation of bioceramics is basically to provide an adequate scaffolding system which helps the bone cells to perform their processes.
Acknowledgements
I would like to express my deepest gratitude to all my co-workers and colleagues that have contributed over the years with their effort and thinking to these studies. Special gratitude is indebted to Pilar Cabañas, Jose Manuel Moreno and Fernando Conde for their help. Financial support by the Spanish CICYT (Mat 2008-00736) is gratefully acknowledged.


