1 Introduction
Besides their synthetic potential, lanthanide trisborohydrides [Ln(BH4)3(THF)3] [1] are used today as efficient catalysts for the polymerization of isoprene [2–5], styrene [4,6] and some polar monomers such as ɛ-caprolactone [7–9], methyl metacrylate [10,11], and trimethylene carbonate [12]. Also the derivatives of [Ln(BH4)3(THF)3] such as mono cyclopentadienyl complexes [3,5,13–16], metallocenes [17,18], alkoxides [13,19,20], guanidinates [21–23] and cyclooctatetraene [15,24] have been used to prepare a large number of lanthanide borohydride derivatives, which also have a high catalytic potential. To obtain these derivatives, [Ln(BH4)3(THF)3] were reacted in a salt metathesis reaction with alkali metal reagents. Beside their catalytic application, borohydride compounds of various metals have also been investigated in general as potential hydrogen storage material [25]. The [Ln(BH4)3(THF)3] were originally prepared in the early 1950s by reaction of rare earth metal alkoxides with B2H6 [26]. In the 1980s, Mirsaidov et al. developed a more convenient approach to obtain [Ln(BH4)3(THF)3] by starting from LnCl3 (Ln = La, Ce, Pr, Nd, Sm) and NaBH4 [27–29].
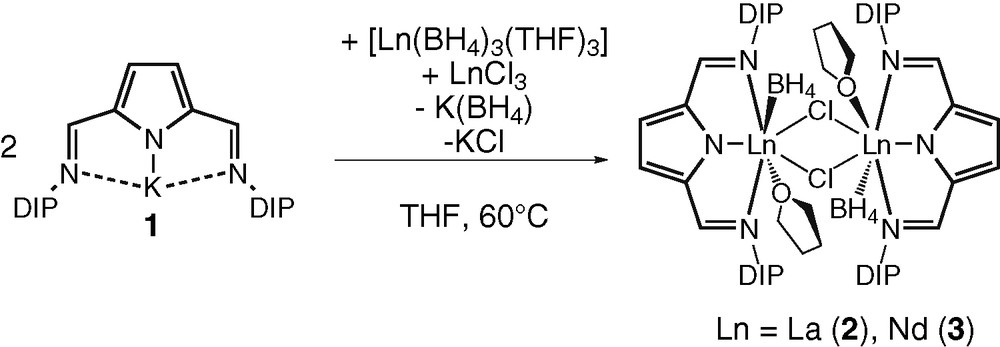
Lately we communicated the reaction of potassium 2,5-bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrolyl [(DIP2-pyr)K] (1) (Scheme 1) with the borohydrides of the rare earth metals, [Ln(BH4)3(THF)3] (Ln = La, Lu) [30]. In dependence of the ionic radius of the center metal atom, the BH4− group reacts either like a pseudo halide or is involved in redox chemistry with the ligand. Thus, the reaction of [(DIP2-pyr)K] with the [La(BH4)3(THF)3], afforded the expected product [(DIP2-pyr)La(BH4)2(THF)2]. In contrast treatment of compound 1 with the borohydrides of the smaller rare earth metal lutetium resulted in a redox reaction of the BH4−group with one of the Schiff-base functions of the ligand. In the isolated product [{(DIP)(DIP-BH3)-pyr}Lu(BH4)(THF)2], a dinegatively charged ligand with a new amido function, a Schiff-base and the pyrrolyl function was bound to the metal atom. The by-product of the reaction of the BH4− anion with the Schiff-base function – a BH3 molecule – was trapped in a unique reaction mode in the coordination sphere of the metal complex.
DIP2-pyr complexes of the lanthanides were also reported by Mashima et al. [31]. This group prepared a number of heteroleptic and homoleptic 2,5-bis(N–aryliminomethyl)pyrrolyl complexes of yttrium by aminolysis of [Y{N(SiMe3)2}3] with the corresponding pyrrolyl ligand. It was shown that the number of pyrrolyl ligands coordinated to a yttrium metal can be controlled by changing the substituents on the aryl rings of the pyrrolyl ligand, resulting in the selective formation of a mono-, bis-, and homoleptic tris(pyrrolyl) complexes [31].
Herein we now report on mixed borohydride-chloride complexes of lanthanum and neodymium having the (DIP2-pyr) ligand in the coordination sphere. Mixed borohydride chloride complexes of the lanthanides are very rare. To the best of our knowledge there are only two reports of structurally characterized compounds. These reports deal with the clusters [(η5–C5Me4nPr)6Ln6(BH4)(12 - x)Clx(THF)n] (x = 10, n = 4, Ln = Sm, Ln = Nd, and x = 5, n = 2, Ln = Sm) [32] and the ionic compound [Li(THF)4]2[Nd2(μ-Cl)2(BH4)6(THF)2] [33].
2 Results and discussion
In order to obtain mixed borohydride-chloride complexes, a 1:1 mixture of [Ln(BH4)3(THF)3] (Ln = La, Nd) and LnCl3 (Ln = La, Nd) was reacted with an equimolar amount of compound 1 in THF at 60 °C. The desired complexes of composition [(DIP2-pyr)LnClBH4]2 (Ln = La (2), Nd (3)) were obtained as yellow single crystals from the mother liquor in good yields (Scheme 1). The new complexes have been characterized by standard analytical/spectroscopic techniques. Compounds 2 and 3 are almost insoluble in THF. Thus, no useful NMR data could be acquired of the diamagnetic compound 2. In the IR spectra of compounds 2 and 3, two characteristic peaks at 2210 cm−1 and 2447 cm−1 (2) and 2229 cm−1 and 2451 cm−1 (3) for each complex were observed, which can be assigned to a terminal coordinated Ln(η3–H3B-H) unit [1].
By crystallizing compounds 2 and 3 from the mother liquor, single crystals were obtained. The solid-state structures of both compounds were established by single crystal X-ray diffraction (Figs. 1 and 2). Both compounds are isostructural to each other. They crystallize in the monoclinic space group C2/c having four molecules in the unit cell. The structures reveal that compounds 2 and 3 are dimeric in the solid state. The metal centers of both compounds are bridged almost symmetrically by two μ-chlorine atoms (La-Cl 2.8695(10) Å, La-Cl′ 2.8733(10) Å(2) and Nd-Cl 2.7984(10) Å, Nd-Cl′ 2.8038(10) Å(3)). Similar structural motives are well established in lanthanide chemistry [34–38]. As expected, a bridging of the metal via the chlorine atoms is preferred. The BH4− groups show a η3-coordination via the hydrogen atoms, which were freely refined for both compounds. This coordination mode is typical for Ln-BH4 compounds [1,16,20,24,29]. The structures reveal a seven-fold coordination sphere spanned by the ligands around the lanthanide atom resulting in a distorted pentagonal bipyramidal coordination polyhedron. In the axis of the distorted pentagonal bipyramid, one THF molecule and one BH4− group are located having a bond angle of O1-La-B 174.65(11)°(2) and O1-Nd-B 173.76(10)°(3). As expected the (DIP2-pyr) ligand is symmetrically attached to the metal center. The metal-imine nitrogen bond distances (La-N1 2.773(3) Å, La-N3 2.758(3) Å (2) and Nd-N1 2.736(3) Å, Nd-N3 2.720(3) Å(3)) are longer than the Nd-Npyrrolyl distance (La-N2 2.438(3) Å (2), Nd-N2 2.393(3) Å). The almost planar arrangement of the LnN3Cl2 fragment is reflected by the sum of the corresponding five valence angles of 357.23°(2) and 358.03°(3).
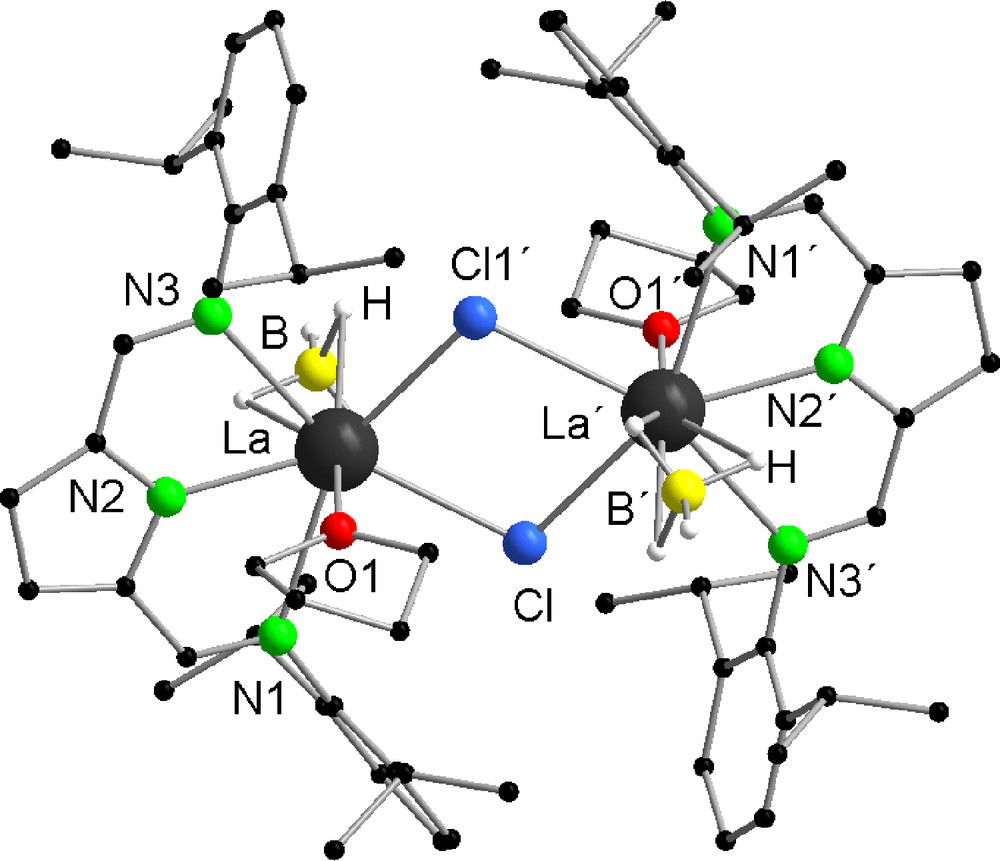
Solid-state structure of 2 showing the atom labeling scheme. The hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: La-N1 2.773(3), La-N2 2.438(3), La-N3 2.758(3), La-O1 2.554(2), La-B 2.682(4), La-Cl 2.8695(10), La-Cl′ 2.8733(10); N1-La-N2 61.73(8), N1-La-N3 123.17(8), N1-La-O1 83.45(8), N1-La-B 93.83(12), N1-La-Cl 80.15(6), N1-La-Cl′ 152.63(5), N2-La-N3 61.44(8), N2-La-O1 75.95(8), N2-La-B 98.70(12), N2-La-Cl 138.94(6), N2-La-Cl′ 136.48(6), N3-La-O1 83.75(8), N3-La-B 93.95(12), N3-La-Cl 152.79(6), N3-La-Cl′ 78.81(6), O1-La-B 174.65(11), O1-La-Cl 85.61(6), O1-La-Cl′ 83.19(6), B-La-Cl 98.49(10), B-La-Cl′ 101.14(10), Cl-La-Cl′ 75.10(3), La-Cl-La′ 104.90(3).
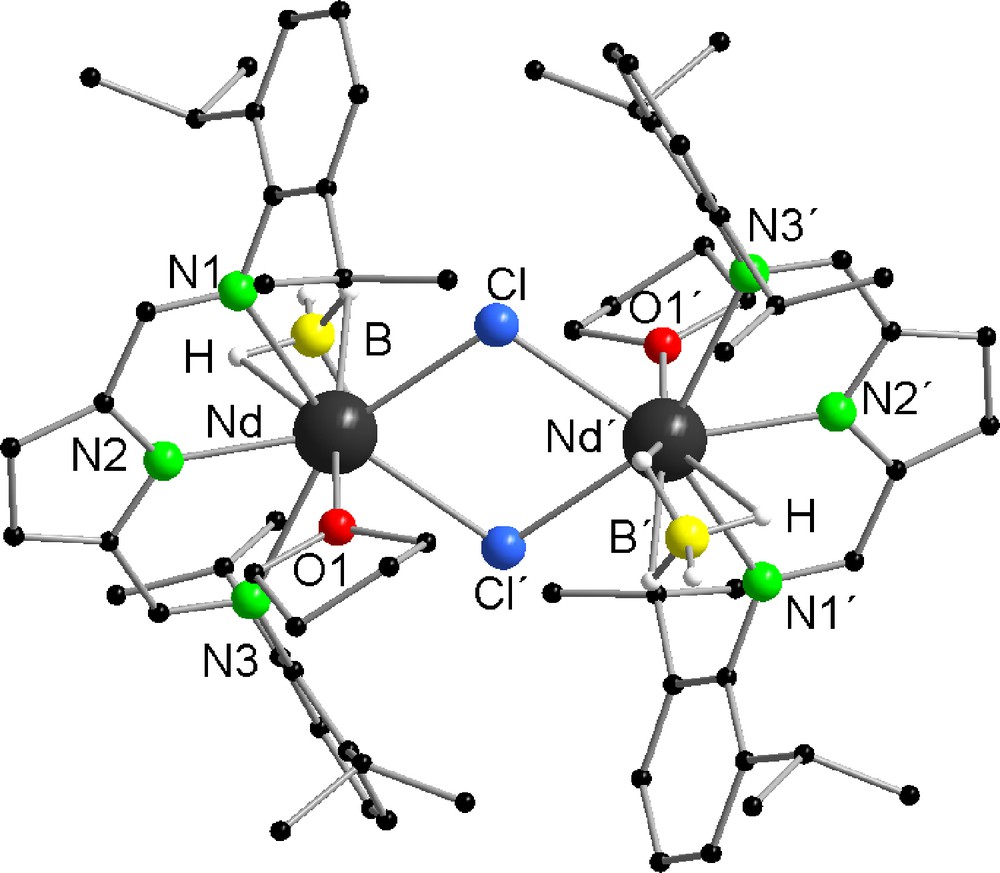
Solid-state structure of 3 showing the atom labeling scheme. Five additional THF solvent molecules and the hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: Nd-N1 2.736(3), Nd-N2 2.393(3), Nd-N3 2.720(3), Nd-O1 2.496(2), Nd-B 2.612(4), Nd-Cl 2.7984(10), Nd-Cl′ 2.8038(10); Nd-Cl-Nd′ 105.02(4), N1-Nd-N2 62.58(8), N1-Nd-N3 125.00(8), N1-Nd-O1 84.26(8), N1-Nd-B 92.98(12), N1-Nd-Cl 79.56(6), N1-Nd-Cl′ 152.57(6), N2-Nd-N3 62.45(9), N2-Nd-O1 76.07(8), N2-Nd-B 97.69(11), N2-Nd-Cl 139.42(7), N2-Nd-Cl′ 137.30(7), N3-Nd-O1 84.63(9), N3-Nd-B 92.43(12), N3-Nd-Cl 152.72(6), N3-Nd-Cl′ 78.46(6), O1-Nd-B 173.76(10), O1-Nd-Cl 86.67(6), O1-Nd-Cl′ 84.24(6), B-Nd-Cl 98.35(10), B-Nd-Cl′ 100.58(10), Cl-Nd-Cl′ 74.98(4).
3 Quantum chemical calculations
To obtain a deeper insight into the bonding in the molecules under discussion, we performed quantum chemical calculations for the lanthanum compound 2. To reduce computational costs, the diisopropylphenyl ligands were replaced by methyl groups. The geometries and energies of this model compound [(Me2pyr)La(THF)ClBH4]2 2′ as well as of its monomeric unit [(Me2pyr)La(THF)ClBH4] 4 were calculated both within the framework of Density Functional Theory (RI-DFT) [39] at the BP86-level [40–42], (basis set of def2-SVP quality for each atom [43,44] and an effective core potential for the inner 46 electrons [45] of La). All the calculations entailed the use of the TURBOMOLE package [46,47]. The electronic structures of 2′ and 4 were calculated under symmetries Ci and C1, respectively. Vibrational frequency calculations at DFT level were performed using the module AOFORCE [48] by diagonalisation of the analytically computed Hessian.
The calculation confirms convincingly the distorted pentagonal bipyramidal coordination sphere spanned by the ligands of the lanthanide atoms in 2′ together with the structural data obtained by the experiment (Table 1). The energy for the dimerisation of the hypothetic monomer is computed to be ΔEdim. = −134 kJ/mol (Etot(2′) = −2452.561878, Etot(4) = −1226.255345 a.u., 1 a.u. = 2625.5 kJ mol−1). The nature of the bonding to lanthanum is estimated by Roby-Davidson-Ahlrichs-Heinzmann population analysis [48]. The calculated shared electron numbers (SEN) as a measure for covalent bonding of the La-O bonds both in 2′ and 4 give rise to a rather weak stabilisation by the THF molecules. Therefore the dimerisation of 4 is favoured over the coordination of another THF molecule. The SEN of the La-Cl bond is reduced significantly going from 4 to 2′ but is still of a typical size for a strongly polar bond.
Comparison of experimental structural data of 2 with those of 2′ and 4 obtained by means of quantum chemical calculations (Å).
| 2 | 2′ | 4 | |||
| r | r | SEN | r | SEN | |
| La-O | 2.554 | 2.660 | 0.22 | 2.659 | 0.13 |
| La-N | 2.438 | 2.497 | 0.45 | 2.490 | 0.48 |
| La-N1 | 2.773 | 2.793 | 0.33 | 2.763 | 0.28 |
| La-Cl | 2.870 | 2.948 | 0.44 | 2.751 | 0.63 |
| La-B | 2.682 | 2.691 | 0.64 | 2.674 | 0.88 |
4 Summary
In summary, we have prepared the mixed borohydride-chloride complexes of lanthanum and neodymium having the DIP2-pyr ligand in the coordination sphere. The title compounds were obtained by the reaction of (DIP2-pyr)K with 1:1 mixture of [Ln(BH4)3(THF)3] (Ln = La, Nd) and LnCl3 (Ln = La, Nd). Both compounds are dimeric in the solid state. The metal atoms are bridged almost symmetrically by two μ-chlorine atoms. The reported compounds are the third class of complexes in rare earth element chemistry with both borohydrides and chloride anions and they are the first lanthanum mixed borohydride-chloride complexes. Quantum chemical calculations for the lanthanum compound 2 were performed to obtain a deeper insight into the bonding in the molecule.
5 Experimental section
5.1 General
All manipulations of air-sensitive materials were performed with the rigorous exclusion of oxygen and moisture in flame-dried Schlenk-type glassware either on a dual manifold Schlenk line, interfaced to a high vacuum (10−3 torr) line, or in an argon-filled MBraun glove box. THF was distilled under nitrogen from potassium benzophenone ketyl prior to use. Hydrocarbon solvents (toluene and n-pentane) were dried using an MBraun solvent purification system (SPS-800). All solvents for vacuum line manipulations were stored in vacuo over LiAlH4 in resealable flasks. IR spectra were obtained by means of an ATR unit on a FTIR Spektrometer Bruker IFS 113 v. Elemental analyses were carried out with an Elementar vario EL. LnCl3 [49], [Ln(BH4)3(THF)3] [15], 2,5-bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrol, [31] and [(DIP2-pyr)K] [50] were prepared according to literature procedures.
5.2 [(DIP2-pyr)LaClBH4]2(2)
THF (20 ml) was condensed at −78 °C onto a mixture of [La(BH4)3(THF)3] (200 mg, 0.50 mmol), LaCl3 (123 mg, 0.50 mmol) and [(DIP2-pyr)K] (1) (480 mg, 0.50 mmol) and the resulting yellow reaction mixture was stirred for 16 h at 60 °C. The yellow solution was concentrated to 10 ml and filtered. The solution was allowed to stand at ambient temperature to obtain the product as pale yellow rhombic crystals after several hours:
- • Yield 343 mg, 0.24 mmol, 49% (single crystals);
- • IR (ν/cm−1): 735 (s), 758 (m), 793 (m), 860 (s), 1012 (m), 1051 (s), 1093 (m), 1159 (s), 1246 (w), 1315 (s), 1335 (s), 1437 (m), 1558 (s), 1651 (w), 1737 (w), 1975 (m), 2031 (m), 2046 (m), 2158 (m), 2210 (m), 2447 (w), 2607 (w), 2860 (w), 2951 (m);
- • (2 + 2 THF) C76H116B2N6O4Cl2La2 (1414.57): calculated C 58.96, H 7.55, N 5.43; found C 58.77, H 7.45, N 5.35.
5.3 [(DIP2-pyr)NdClBH4]2(3)
THF (20 ml) was condensed at −78 °C onto a mixture of [Nd(BH4)3(THF)3] (171 mg, 0.42 mmol), NdCl3 (106 mg, 0.42 mmol) and [(DIP2-pyr)K] (1) (405 mg, 0.84 mmol) and the resulting yellow reaction mixture was stirred for 16 h at 60 °C. The yellow solution was filtered off and concentrated until a yellow precipitate appears. The mixture was heated carefully until the solution became clear. The solution was allowed to stand at ambient temperature to obtain the product as yellow crystals after several hours.
- • Yield 250 mg, 0.18 mmol, 42% (single crystals).
- • IR (KBr, ν/cm−1): 734 (s), 802 (m), 860 (m), 1012 (m), 1052 (s), 1098 (m), 1163 (s), 1254 (w), 1344 (s), 1440 (m), 1563 (vs), 1731 (w), 1801 (w), 1862 (w), 2048 (m), 2079 (m), 2105 (m), 2229 (w), 2318 (w), 2451 (w), 2654 (w), 2863 (w), 2953 (m).
- • (3) C68H100B2N6O2Cl2Nd2 (1414.57): calculated C 57.74, H 7.13, N 5.94; found C 58.32, H 7.24, N 5.56.
5.4 X-ray crystallographic studies of 2 and 3
Crystals of 2 and 3 were grown from THF. A suitable crystal of compounds 2 and 3 was covered in mineral oil (Aldrich) and mounted onto a glass fiber. The crystal was transferred directly to the −73 °C or −123 °C N2 cold stream of a Stoe IPDS 2 diffractometer. Subsequent computations were carried out on a Core2Duo.
All structures were solved by the Patterson method (SHELXS-97 [51]). The remaining non-hydrogen atoms were located from successive difference Fourier map calculations. The refinements were carried out by using full-matrix least-squares techniques on F2, minimising the function (Fo − Fc)2, where the weight is defined as and Fo and Fc are the observed and calculated structure factor amplitudes using the program SHELXL-97 [51]. Carbon-bound hydrogen atom positions were calculated and allowed to ride on the carbon to which they are bonded. The hydrogen atom contributions of all compounds were calculated, but not refined. The locations of the largest peaks in the final difference Fourier map calculation as well as the magnitude of the residual electron densities in each case were of no chemical significance.
2 (5 THF) C88H140B2Cl2La2N6O7, monoclinic, C2/c (no. 15) lattice constants a = 29.055(6), b = 14.167(3), c = 23.347(5) Å, β = 106.16(3), V = 9230(3) Å3, Z = 4, μ(Mo-Kα) = 1.023 mm−1, θmax = 26.73°, 9800 [Rint = 0.0712] independent reflections measured, of which 8166 were considered observed with I > 2σ(I); max. residual electron density 2.404 and −0.880 e/A–3; 436 parameters, R1 (I > 2σ(I)) = 0.0402; wR2 (all data) = 0.1103.
3 (5 THF) C88H140B2Cl2N6Nd2O7, monoclinic, C2/c (no. 15) lattice constants a = 29.057(6), b = 14.147(3), c = 23.195(5) Å, β = 106.47(3), V = 9144(3) Å3, Z = 4, μ(Mo-Kα) = 1.234 mm−1, θmax = 27.93°, 10928 [Rint = 0.0379] independent reflections measured, of which 9273 were considered observed with I > 2σ(I); max. residual electron density 2.445 and −1.275 e/A−3; 436 parameters, R1 (I > 2σ(I)) = 0.0417; wR2 (all data) = 0.1106.
Positional parameters, hydrogen atom parameters, thermal parameters, bond distances and angles have been deposited as supporting information. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as a supplementary publication no. CCDC- 752139-752140. Copies of the data can be obtained free of charge on application to CCDC, 12 Union road, Cambridge CB21EZ, UK (fax: +(44)1223-336-033; email: deposit@ccdc.cam.ac.uk).
Acknowledgement
This work was supported by the Deutsche Forschungsgemeinschaft (SPP 1166). R.K. gratefully acknowledges the Leibniz-Rechenzentrum München (HLRB project h1191) for computational resources.


