1 Introduction
Developing a simple, eco-friendly as well as economic reaction protocol for the synthesis of organic transformations is an attractive area of research in both academic and pharmaceutical R and D [1]. Hence the challenging task of achieving ‘efficiency’ in all aspects of chemical production, advocated by green chemistry can be realized by innovative research which comprehensively addresses the issues of atom economy, economy and avoidance of auxiliary chemicals [2]. By keeping these ideas in mind and paraphrasing the concept reported by Sheldon “the best solvent is no solvent” [3] and by Maggi “the best catalyst is no catalyst” [4], we can state that the greener protocol is one which involves no catalyst and no solvent or use of water as a universal solvent.
Xanthenes are frequently occurring motifs in a number of natural products [5], and have been used as versatile synthons due to the inherent reactivity of the inbuilt pyran ring [6] Most of the natural schweinfurthins [7] (diversonol and blennolide C, Fig. 1) are potent and selective inhibitors of cell growth as measured by the National Cancer Institute's 60-cell line screen [8]. Xanthenes are known for their utility as leuco-dyes [9], pH-sensitive fluorescent materials for the visualization of biomolecules [10] and in laser technologies [11] due to their useful spectroscopic properties. Octahydroxanthene derivatives containing a structural unit of benzopyrans can be used as antispasm [12] and fluorescent fuel [13]. These multifarious applications of xanthenediones have stimulated both the development of environmentally benign methods for their synthesis as well as their derivatives.

Important xanthene derivatives.
In contrast to the widely studied 1,8-dioxo-octahydroxanthene derivatives [12,13], relatively few papers describe the chemistry of 1-oxo-hexahydroxanthenes [14–17]. Synthetic routes to 1-oxo-hexahydroxanthenes generally involve prolonged heating in acid-catalyzed reactions of salicylaldehyde with 1,3-diketone. The classical methods involve catalysts viz 2,4,6-trichloro-1,3,5-triazine (TCT) [14], KF/ Al2O3 [15], CeCl3.7H2O [16] and triethylbenzylammonium chloride (TEBA as a cationic surfactant) [17]. Therefore an environmentally benign protocol for synthesis of 1-oxo-hexahydroxanthenes is highly desirable. In view of this, we are working on the synthesis of 1-oxo-hexahydroxanthenes using an eco-friendly synthetic strategy. Hence we report our findings.
2 Results and discussion
EPZ-10, a clay catalyst commercially available from Contract Chemicals, England (www.contract-chemicals.com.), which is prepared by supporting ZnCl2 on clay, is known to contain predominantly strong Lewis acid sites as well as weak Bronsted acid sites [18]. Recently, we have reported synthesis of 1,8-dioxo-octahydroxanthenes in aqueous medium using EPZ-10 (10 mol %) as an ecofriendly catalyst under reflux conditions [19]. This spurned us to investigate the capability of EPZ-10 to act as catalyst for the synthesis of 1-oxo-hexahydroxanthene. Initially the reaction of salicylaldehyde (1, R’ = H) and dimedone (2, R = Me) [1:2] was carried using EPZ-10 (10 mol %) in aqueous medium at reflux conditions and corresponding 1-oxo-hexahydroxanthene (3) (Scheme 1) was obtained in 92% yield in 3 h. In order to investigate the role of EPZ-10, the above reaction was performed in the absence of the catalyst and gratifyingly 1-oxo-hexahydroxanthene was achieved in 90% yield. After this success, we focused our attention towards catalyst-free synthesis of 1-oxo-hexahydroxanthene in aqueous medium.
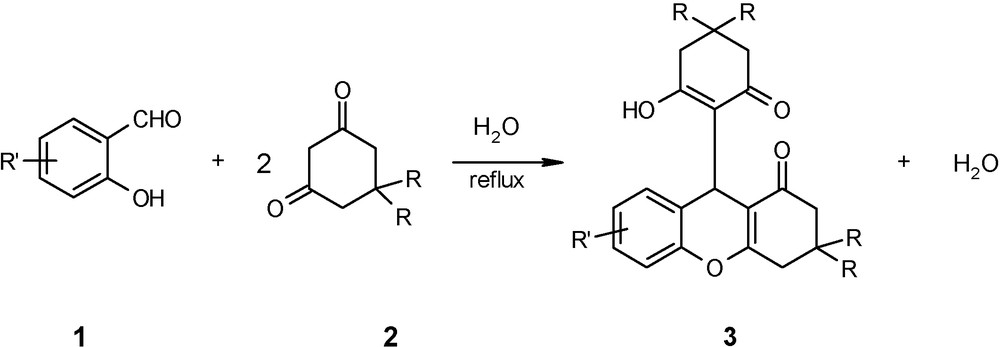
Catalyst-free synthesis of 1-oxo-hexahydroxanthenes.
Using the catalyst-free condition, both electron-rich as well as electron-deficient salicylaldehydes reacted effectively with 1,3-dicarbonyl compounds in aqueous medium. (Table 1) Both dimedone and cyclohexane-1,3-dione reacted smoothly with substituted salicylaldehydes such as 5-chloro-, 3-methoxy-, 5-bromo, 4 and 5-hydroxy, 3,5-dichloro and 3,5-dibromo derivatives to produce the corresponding 1-oxo-hexahydroxanthene in high yields (Table 1, entries a–o).
Catalyst-free synthesis of 1-oxo- hexahydroxanthenes.
| Entry | Product (3) | Time (h) | Yield (%)a,b | M.P Obs.(Lit oC) |
| a | R = H; R’ = CH3 | 3.5 | 90 | 204 (206) [14] |
| b | R = 5-Cl ; R’ = CH3 | 5.0 | 88 | 235 (238) [14] |
| c | R = 3-OMe; R’ = CH3 | 4.5 | 85 | 231 (230) [14] |
| d | R = 5-Br ; R’ = CH3 | 5.0 | 86 | 251 (253) [14] |
| e | R = 5-OH; R’ = CH3 | 4.0 | 93 | 217 (------) |
| f | R = 4-OH; R’ = CH3 | 4.0 | 95 | 232 (------) |
| g | R = 3,5 Cl; R’ = CH3 | 4.5 | 87 | 231 (235) [14] |
| h | R = H; R’ = H | 3.5 | 88 | 242 (244) [14] |
| i | R = 3,5 Cl; R’ = CH3 | 4.0 | 85 | 252 (254) [14] |
| k | R = 5-NO2; R’ = H | 5.0 | 87 | 243 (245) [14] |
| l | R = 3,5-Br; R’ = H | 5.0 | 89 | 254 (255) [14] |
| m | R = 5-OH; R’ = H | 4.5 | 89 | 227 (------) |
| n | R = 4-OH; R’ = H | 4.5 | 91 | 240 (------) |
| o | R = H; R’ = H | 4.5 | 84 | 215 (216) [14] |
In recent years, water has been demonstrated as an ideal medium for many organic transformations even if starting materials and products appear to be insoluble [20]. Many organic reactions such as Claisen rearrangement, aldol condensation, benzoin condensation, and Diels–Alder cycloaddition exhibit rate enhancement in water [21].
In this reaction water (ɛ = 78.6) helps in the easy conversion of the keto form to enol form of the compound (2) which is useful for Knoevenagel condensation followed by subsequent Michael addition and dehydration affords product (3). A plausible mechanism is depicted in Scheme 2.
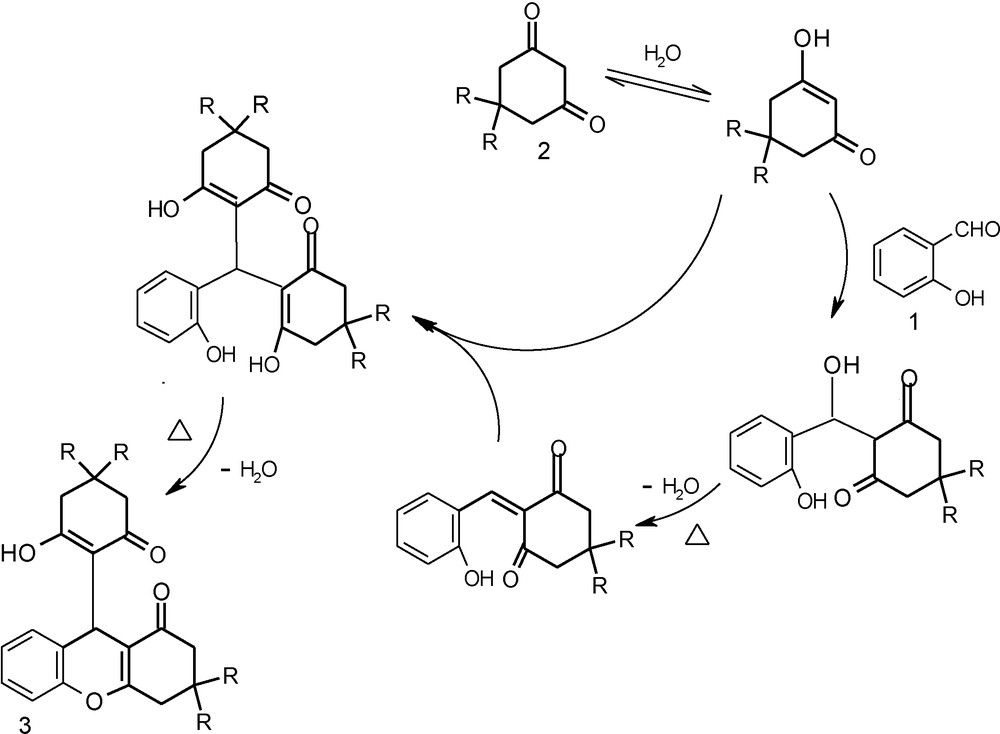
A mechanism for formation of 1-oxo-hexahydroxanthene influenced by water.
3 Conclusion
In conclusion, we have developed practical and eco-friendly method for the efficient synthesis of 1-oxo-hexahydroxanthenes by a condensation of salicylaldehyde and dimedone/cyclohexane-1,3-dione. The use of water as a solvent, simple work-up procedure and no need of catalyst make it an eco-friendly as well as an economical alternative to earlier reported approaches.
4 Experimental
Various substituted salicylaldehydes (Sigma-Aldrich), 1,3-diketones viz cyclohexane-1,3-dione (Alfa Aesar) and dimedone (Thomas Baker) were used as received. Melting points were determined and are uncorrected. IR spectra were recorded on Perkin-Elmer [FT-IR-783] spectrophotometer. NMR spectra were recorded on Bruker AC-300 (300 MHz for 1H NMR and 75 MHz for 13C NMR) spectrometer in DMSO-d6 or CDCl3 using TMS as an internal standard and δ values are expressed in ppm. Mass spectra (LCMS) were recorded on Shimadzu LCMS 2010, mass spectrometer.
4.1 General procedure for catalyst-free synthesis of 1-oxo-hexahydroxanthenes
A suspension of an salicylaldehyde (1 mmol) and dimedone/cyclohexane-1,3-dione (2 mmol) in water (5 mL) were stirred at reflux condition and the progress of the reaction was monitored by TLC. After completion of the reaction, the resulting solid products were collected by filtration and were recrystallized from 95% ethanol. These products were characterized by usual spectral techniques. (i.e. IR, 1H and 13C NMR, LCMS).
4.2 Spectral data of unknown compounds:
Compound (3 e); mp. 217 oC, IR (KBr): 3110, 2958, 2926, 1589, 1468, 1382, 1230, 1192, 1034, 820 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 10.29 (bs, 1H), 9.07 (s, 1H), 6.74 (d, 1H, J=8.7 Hz), 6.45 (dd, 1H, J=8.7 Hz and J=2.7 Hz), 6.37 (d, 1H, J=2.7 Hz), 4.95 (s, 1H), 1.96-2.49 (m, 8H), 1.01 (s, 3H), 0.95 (s, 3H), 0.88 (s, 6H); 13CMR: 26.64, 29.69, 32.01, 41.21, 50.90, 99.98, 110.38, 114.10, 114.37, 116.39, 116.78, 126.72, 143.12, 154.10, 165.40, 195.54, 196.12; LCMS: m/z 382.
Compound (3 f); mp. 232 oC IR (KBr): 3308, 3196, 2955, 2927, 1621, 1457, 1382, 1274, 1145, 1034, 845 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 10.25 (bs, 1H), 9.37 (s, 1H), 6.72 (d, 1H, J=8.4 Hz), 6.39 (dd, 1H, J=8.4 Hz and J=1.5 Hz), 6.29 (d, 1H, J=1.5 Hz), 4.90 (s, 1H), 1.96-2.48 (m, 8H), 1.01 (s, 3H), 0.95 (s, 3H), 0.86 (s, 6H); 13CMR: 26.67, 28.17, 29.64, 32.03, 50.90, 99.88, 102.28, 111.60, 112.21, 116.34, 129.22, 150.48, 156.54, 165.07, 195.27, 196.27; LCMS: m/z 382.
Compound (3 m); mp. 227 oC IR (KBr): 3346, 2948, 2918, 1622, 1598, 1450, 1379, 1299, 1196, 1002, 920 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 10.39 (bs, 1H), 9.08 (s, 1H), 6.75 (d, 1H, J=8.7 Hz), 6.46 (dd, 1H, J=8.7 Hz and J=2.7 Hz), 6.39 (d, 1H, J=2.7 Hz), 4.98 (s, 1H), 1.67-2.48 (m, 12H); 13CMR: 20.77, 20.86, 26.12, 27.81, 37.14, 99.99, 111.57, 114.12, 114.40, 116.33, 119.72, 126.82, 143.09, 154.09, 167.30, 196.36; LCMS: m/z 326.
Compound (3 n); mp. 240 oC, IR (KBr): 3339, 3190, 2954, 1624, 1589, 1456, 1378, 1231, 1189, 1139, 987 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 10.35 (bs, 1H), 9.38 (s, 1H), 6.74 (d, 1H, J=8.1 Hz), 6.39 (dd, 1H, J=8.1 Hz and J=2.1 Hz), 6.29 (d, 1H, J=2.1 Hz), 4.92 (s, 1H), 1.65-2.21 (m, 12H); 13CMR: 20.75, 20.85, 25.21, 27.73, 37.13, 99.98, 102.19, 112.27, 112.80, 116.45, 120.00, 129.26, 150.55, 156.55, 166.96, 196.54; LCMS: m/z 326.
Acknowledgements
Authors DMP and KAU thank UGC, New Delhi for financial assistance [F.2-3/2007(Policy/SR)] and for the Research Fellowship, respectively.


