Chiral sulfoxides are used as auxiliaries in asymmetric synthesis and also as ligands in enantioselective catalysis (for reviews on the use of chiral sulfoxides: [1]). Moreover, a number of pharmaceutically important drugs contains asymmetric sulfinyl moieties (for a general overview on asymmetric syntheses of biologically active chiral sulfoxides: [2]).1 Enantioselective oxidations of sulfides can be performed either by biocatalysis [3,4] or by chemical oxidation, the latter occurring in the presence of chiral oxidising species, or by using achiral oxidants and chiral metal complexes [5–9].
The asymmetric oxidation of sulfides by metal catalysts is one of the most attractive routes to optically active sulfoxides, so far reported [10], and tungsten-based catalysts have been shown to succeed in the oxidation of sulfides to sulfoxides [11].
Combining the areas of chiral tungsten catalysts with tailored or functionalised ionic liquids, a new class of chiral ionic liquids [12] has been prepared, in which the catalytic metal centre and chirality are focussed in the anion and the cation was chosen for its imparted hydrophobicity. These ionic liquids were prepared incorporating trihexyltetradecylphosphonium [P6 6 6 14]+ and methyltrioctylammonium [N1 8 8 8]+ cations to improve organic miscibility. These ionic liquids were synthesised using tungstate(VI) complexes containing (S)-mandelic acid (Hmand) and (S)-1,1′-binaphthol (H2binol) as chiral ligands. To the best of our knowledge, these anions have not been utilised previously for promoting chiral sulfide oxidations. Enantioselective oxidation of sulfides to sulfoxides, using hydrogen peroxide or urea hydrogen peroxide (UHP) [13] (and references therein) as the sacrificial oxidant, was demonstrated with good yields and selectivity, with enantiomeric excesses up to 96%, using the ionic liquids as chiral catalysts. These results indicate that efficient chiral induction was promoted by the presence of both the catalytic site and the stereogenic centre in the anion.
Two new salts containing the tungsten(VI)-2,2′-(S)-binaphthol complex were prepared via a two-step process. The first step involved preparation of the salt Na[N4 4 4 4][WO2(S-binol)2] [14] and in the next step cation exchange was performed by a simple metathesis in either CH2Cl2 or CHCl3 with [P6 6 6 14]Cl, or [N1 8 8 8]Br. The removal of tetraalkylammonium halides yielded [P6 6 6 14]2[WO2(S-binol)2] and [N1 8 8 8]2[WO2(S-binol)2] (Fig. 1) as pale yellow solids possessing low melting points (54 °C and 116–117 °C, respectively). Similarly, two other ionic liquids containing a tungsten(VI)-(S)-mandelate complex were prepared via Na2[WO2(S-mand)2] [15] following a similar experimental procedure. [P6 6 6 14]2[WO2(S-mand)2] and [N1 8 8 8]2[WO2(S-mand)2] were obtained (Fig. 1). All the ionic liquids were characterised by 1H and 13C NMR spectroscopy, ESI-mass spectrometry, DSC, CD spectroscopy and microanalysis. [P6 6 6 14]2[WO2(S-mand)2] was a room temperature ionic liquid with a broad glass transition (DSC), whereas [N1 8 8 8]2[WO2(S-mand)2] was a solid (m.p. 69 °C) at room temperature (salts that possess melting points below 100 °C are categorised as ionic liquids: [16]). The catalytic activity of these ionic liquids was tested in a model reaction, oxidation of phenyl methyl sulfide (Scheme 1).

Structures of anions used in this study.
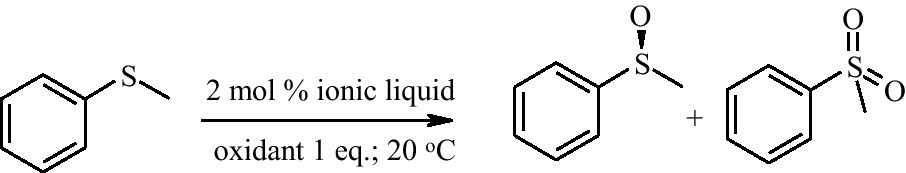
Model test reaction.
The reaction was screened using [P6 6 6 14]2[WO2(S-binol)2] as catalyst in a range of solvents with UHP or aqueous hydrogen peroxide as oxidants (Table 1). When the reaction was performed using 2 mol % of this catalyst at room temperature in CH2Cl2, with 1.15 equivalent of UHP, after 6 h just 5% of sulfoxide with 95% of ee was obtained (Table 1, entry 1) [17].
Enantioselective oxidation of methyl phenyl sulfide (Scheme 1) catalysed by 2 mol % [P6 6 6 14]2[WO2(S-binol)2].
| Entry | Solvent | Oxidant | Sulfoxide yield/%a | Select./% | ee/%b |
| 1 | CH2Cl2 | UHP | 5 | 100 | 95 |
| 2 | Solventless | UHP | 58 | 50 | 6 |
| 3 | [bmpyrr][NTf2] | UHP | 29 | 60 | 10 |
| 4 | [C6mim]Cl | UHP | 62 | 75 | 5 |
| 5 | H2O | H2O2 (30%) | 12 | 100 | 25 |
| 6 | aq. Na[AOT]c | H2O2 (30%) | 64 | 82 | 6 |
a Yield determined by HPLC.
b ee value determined by chiral HPLC.
c 5 mol %, 25 mM (sodium diisooctylsulfosuccinate).
Similar results were obtained using aqueous H2O2 (30%). Under the same reaction conditions, using UHP as oxidant [N1 8 8 8]2[WO2(S-binol)2] was tested as a catalyst, but changing the cation made no difference to the catalytic activity. With the aim of increasing the sulfide conversion, the reaction was performed in absence of solvent, using both the oxidants. With UHP as oxidant, 58% of sulfide conversion was observed with 50% of selectivity and 6% of enantiomeric excess after 4 h (Table 1, entry 2). Similar results were obtained using aqueous H2O2 (30%).
The reaction was then performed using the ionic liquids 1-butyl-1-methylpyrrolidinium bis{(trifluoromethyl)sulfonyl}amide ([C4mpyrr][NTf2]) or 1-hexyl-3-methylimidazolium chloride ([C6mim]Cl) as solvent and UHP as oxidant. Low selectivities and enantiomeric excesses were obtained (Table 1, entries 3 and 4). Using water as a solvent and aq. H2O2 (30%) as oxidant, after 1 h, 12% sulfoxide was obtained in a 25% enantiomeric excess (Table 1, entry 5). Adding Na[AOT], as a surfactant phase transfer reagent (AOT = bis(2-ethylhexyl) sulfosuccinate), an increase of conversion was observed with a decrease of the enantiomeric excess (Table 1, entry 6). [P6 6 6 14]2[WO2(S-binol)2] was shown to promote the oxidation of sulfide to sulfoxide with low enantioselectivity.
In order to study the effect of changing the chiral ligand on the catalytic ability of these ionic liquids, the catalytic activity of [P6 6 6 14]2[WO2(S-mand)2] was tested in the model reaction. A preliminary study was performed using Na2[WO2(S-mand)2] as catalyst at room temperature, aq. H2O2 (30%) or UHP as oxidant, in MeOH. After 1 h, high sulfide conversion was observed, 91% and 89% respectively, with 100% of selectivity in both cases, but low enantiomeric excess (17%).
Using dichloromethane instead of methanol, and H2O2 (30%) as oxidant, a high enantiomeric excess was observed, ≥ 98%, although only a low sulfoxide yield of 12% was obtained. Furthermore, the sodium salt is not completely soluble in CH2Cl2. In order to increase the sulfide conversion without compromising the excellent enantiomeric excess, the reaction was performed using the hydrophobic ionic liquid, [P6 6 6 14]2[WO2(S-mand)2] instead of the sodium salt. With a chiral tungstate(VI) complex in ionic liquid form, a completely homogeneous system was obtained. As shown in the Tables 2 and 3, using this system, a high enantiomeric excess with good conversion and selectivity was achieved. With UHP as oxidant in CH2Cl2, only 10% of sulfide conversion to sulfoxide with 95% enantiomeric excess was obtained, after 1 h (Table 2, entry 1). Using 30% H2O2 as oxidant, 44% of sulfide conversion and 30% enantiomeric excess was obtained (Table 2, entry 2). UHP is the oxidant that gave the highest enantiomeric excess. The slow release of H2O2 from UHP appears to play an important role in this oxidation. As clearly shown in Table 2, the change in the form of oxidant from 30% aq. H2O2 to UHP resulted in a dramatic increase of the ee. In order to increase the sulfide conversion, without compromising the high enantiomeric excess, the amount of the catalyst was increased to a desired level.
Effect of the nature of oxidant on enantioselective oxidation of methyl phenyl sulfide (Scheme 1) catalysed by [P6 6 6 14]2[WO2(S-mand)2].
| Entry | Oxidant | Sulfoxide yield/%a | Select/% | ee/%b |
| 1 | UHP | 10 | 100 | 95 |
| 2 | aq. H2O2 (30%) | 44 | 100 | 30 |
a Yield determined by HPLC.
b ee value determined by chiral HPLC.
Effect of the amount of catalyst, [P6 6 6 14]2[WO2(S-mand)2], on enantioselective oxidation of methyl phenyl sulfide (Scheme 1).
| Entrya | Cat./mol % | Sulfoxide yield/%b | Select./% | ee/%c |
| 1 | 1 | 10 | 100 | 95 |
| 2 | 2 | 36 | 78 | 95 |
| 3 | 5 | 58 | 82 | 95 |
a UHP 1.15 eq., room temperature, CH2Cl2, 1 h.
b Yield determined by HPLC.
c ee value determined by chiral HPLC.
Using UHP as oxidant in CH2Cl2 and increasing the amount of catalyst from 1 to 5%, the sulfoxide yield increased from 10 to 58% with high enantiomeric excess (95%). A good selectivity was also maintained, as shown in Table 3.
Improved results were also obtained adding a small percentage of ethanol, 2%, to CH2Cl2 and using just 1% of [P6 6 6 14]2[WO2(S-mand)2] as catalyst, the sulfide conversion increased from 10 to 41%, with 98% of selectivity and 88% of enantiomeric excess (Table 4, entry 2).
Solvent effects on enantioselective oxidation of methyl phenyl sulfide (Scheme 1) with [P6 6 6 14]2[WO2(S-mand)2].
| Entrya | Solvent | Cat./mol % | Sulfoxide yield/%b | Select./% | ee/%c |
| 1 | CH2Cl2 | 1 | 10 | 100 | 95 |
| 2 | CH2Cl2-2% EtOH | 1 | 41 | 98 | 88 |
| 3 | thf | 1 | 85 | 100 | < 10 |
| 4 | MeOH | 1 | 93 | 84 | < 10 |
| 5 | Cyclohexane | 1 | 8 | 67 | < 10 |
| 6 | [C4mpyrr][NTf2] | 1 | 51 | 84 | < 10 |
| 7 | H2O | 1 | 94 | 96 | < 10 |
| 8 | aq. Na[AOT] | 1 | 86 | 90 | < 10 |
| 9 | Solventless | 1 | 76 | 85 | < 10 |
| 10d | CH2Cl2-2% EtOH | 1 | 53 | 95 | 96 |
| 12 | CH2Cl2 | 5 | 58 | 82 | 95 |
a UHP 1.15 eq., room temperature, 1 h.
b Yield determined by HPLC.
c ee value determined by chiral HPLC.
d 10 oC/3 h.
As expected, decreasing the reaction temperature to 10 °C led to an increase of the enantiomeric excess to 96% with 53% sulfoxide yield and 95% selectivity (Table 4, entry 10). However, a further increase of the reaction time did not give better results. Different solvent systems such as tetrahydrofuran (thf), methanol, cyclohexane, [C4mpyrr][NTf2], water and water with an added phase transfer reagent were also tested, giving unsatisfactory results. CH2Cl2-2% EtOH is the solvent system that gave the best results, as shown in Table 4.
Over a longer reaction time, an increase in the sulfide conversion was observed associated with high selectivity, but resulting in a drop of the enantiomeric excess. It is possible that the increased amount of water generated in the system may have an effect on the tungsten(VI) complex anion. Also the solvents would play an important role in organising the substrate at the ligating site of the complex.
In conclusion, a new class of catalytic ionic liquids containing a tungsten(VI) metal centre bearing chiral ligands has been prepared. These ionic liquids are shown to promote enantioselective oxidation of sulfides to sulfoxides with hydrogen peroxide related oxidants. A simple protocol for achieving high enantioselectivities and conversions is presented. The versatility of bulky and hydrophobic [P6 6 6 14]+ and [N1 8 8 8]+ cations for constructing ionic liquids with complex catalytic anions is also demonstrated. A detailed full paper on recyclability and use of other similar catalytic systems will be published elsewhere. These types of ionic liquids containing other desirable metals could pave the way for further developments in other forms of oxidations. The synthesis of chiral catalytic ionic liquids for other enantioselective oxidation reactions, such as epoxidation is currently under way.
Acknowledgment
K.R.S acknowledges EPSRC (Portfolio Partnership Scheme, grant number EP/D029538/1). C.Q. wishes to thank the Marie-Curie Foundation for providing a fellowship for her to work at QUILL. We acknowledge Dr A. Robertson at Cytec for providing phosphonium salts. Dr J.D. Holbrey is acknowledged for his valuable discussions and Angela Brownlie for her technical support.
1 One of the most popular drugs in the world (with total sales in 2002 of US$ 6.6 billion) is the chiral sulfoxide Omeprazole (used in the treatment of dyspepsia, peptic ulcer disease [PUD], gastroesophageal reflux disease [GORD/GERD] and Zollinger-Ellison syndrome). Its enantioselective synthesis involves an asymmetric sulfide oxidation (for a summary of recent developments and data: [2]).


