1 Introduction
Catalysis in the synthesis of fine chemicals is an important area offering environmentally more acceptable processes. From an industrial point of view, heterogeneous catalysis is generally preferred to homogeneous catalysis mainly because of the easy recovering and possible recycling of the catalyst. However, in various examples, limitations of mass or heat transfer lead to decreased activity. Furthermore, lower selectivities are often obtained [1]. To circumvent these limitations, heterogeneisation of homogeneous catalysts has led to the development of new catalytic systems based on supported liquid phase. Among them, supported ionic liquid catalysts (SILC) on inorganic material (SiO2, Al2O3) are mainly encountered [2]. Natural polymers, particularly polysaccharides, which fulfill most of the requisite properties for a support: (enormous quantities on Earth, insolubility in the majority of organic solvents, high affinity for ionic liquids, high chemical stability and biodegradability) are, in comparison, neglected [3] (for some recent use of biopolymers as catalyst support, see [3]). We have recently developed original SILC based on a chitosan supported ionic liquid [4] phase and demonstrated their high activity and efficient recycling in the benchmark Tsuji-Trost allylation reaction, one of the most important reactions for carbon-carbon and carbon-heteroatom bonds formation [5,6]. In order to evaluate the influence of the chemical structure of the support on the catalytic properties of the SILC, we have extended our work to another biopolymer: the alginates. In this article, the preparation of new supported ionic liquid phase catalysts based on alginate is reported and the activity of these new catalytic materials is compared with that obtained with chitosan-supported ionic liquid phase catalysts.
2 Experimental
2.1 General comments
The ionic liquid [bmim][BF4] was supplied by Solvionic (Toulouse, France). All commercially available compounds were used as received. Thin layer chromatography was carried out on silica gel 60 F254 (1.1 mm, Merck) with spot detection under UV light or through KMnO4 oxidation. Chromatographic separations were achieved on Merck silica gel column (Geduran Si 60, 0.040–0.063 nm).
2.2 Preparation of biopolymer beads
2.2.1 Alginate beads
Sodium alginate (Acros) was dissolved in distilled water at a concentration of 1% (w/w). The polymer solution was added dropwise at room temperature to the stirred CaCl2 solution (0.25 M) using a syringe. The beads were cured in the gelation solution for 3 h. They were rinsed with water and freeze-dried.
2.2.2 Chitosan beads
1 g of chitosan (Fluka) characterised by an average molar mass of 330,000 g.mol−1 (determined by viscosimetry) and a degree of deacetylation determined by 1H NMR of 80%, was dissolved in 100 mL of a 0.2% HCl solution. After complete dissolution, the solution was filtered on a Büchner, and dropped into a NaOH solution (0.25 mol.L−1) through a syringe needle. Chitosan beads were rinsed with water until reaching water conductivity. Beads were then freeze-dried.
2.3 Preparation of biopolymer-SILC
Predegassed [bmim][BF4] was added to Pd(OAc)2 (0.02 mmol, 0.05 equiv.), trisulfonated triphenyl phosphine trisodium salt (TPPTS ligand) (0.079 mmol, 0.2 equiv.) and the biopolymer beads (100 mg of chitosan or 50 mg of alginate). An optimum amount of 0.5 mL of [bmim][BF4], which corresponds to the maximum quantity of IL which can be absorbed by the beads, was used for chitosan-SILC and 0.3 mL for alginate-SILC. The resulting mixture was stirred 30 min at room temperature under inert atmosphere.
2.4 General procedure for the Pd-catalysed allylic substitution of (E)-1,3-diphenyl-3-acetoxyprop-1-ene with dimethyl malonate
(E)-1,3-diphenyl-3-acetoxyprop-1-ene (100 mg, 0.40 mmol, 1 equiv.), AcOK (0.02 mmol, 0.05 equiv.), N,O-Bis(trimethylsilyl)acetamide (BSA) (1.19 mmol, 3 equiv.) and dimethyl malonate (1.19 mmol, 3 equiv.) were added to the biopolymer-SILC previously prepared. The mixture was stirred at room temperature under inert atmosphere and the reaction monitored by TLC. Products were extracted with 10 × 1 mL of ether for chitosan and 15 × 1 mL of ether for alginate (monitored by TLC). The combined organic layers were washed with water, dried over MgSO4, filtered and concentrated under reduced pressure. Then, the obtained residue was purified by column chromatography on silica gel (petroleum ether/ether: 9/1). After the extraction of the product, the biopolymer-SILC was dried under vacuum and reused for another catalytic cycle.
3 Results and discussion
Alginates are produced by brown algae and mainly consist of (1→ 4) linked β-D-mannuronic (M) and α-L-guluronic residues (G) (Fig. 1a). Alginates differ one from another only by their M/G ratio. The use of alginates as catalytic support is recent and mainly lies in their ability to form heat-stable strong gel with divalent cations, especially Ca2+ ([7] and references cited therein). They have been largely used for the entrapment of biologically active materials ([8] and references cited therein).
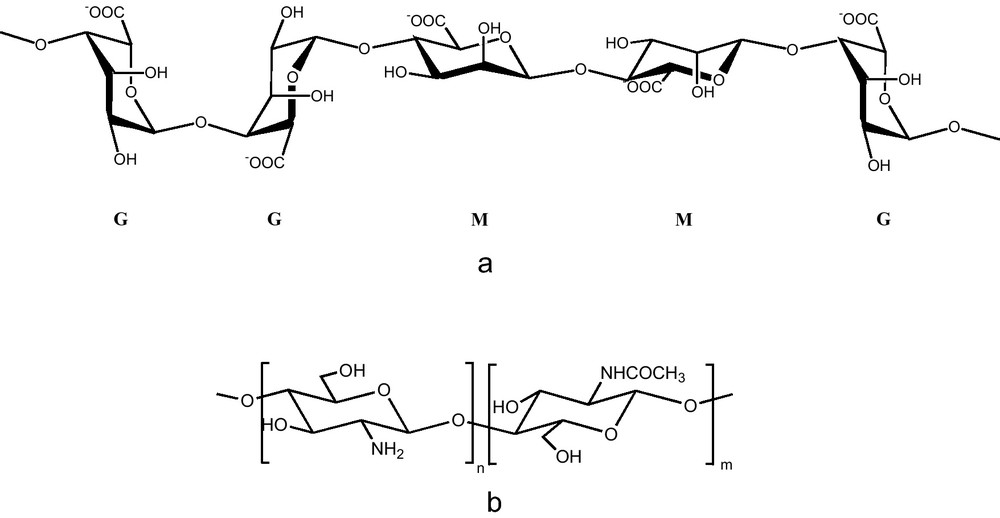
a: the blocks of mannuronate (M) and guluronate (G) residues, which form the alginate; b: chemical structure of chitosan.
Chitosan, which consists of 2-amino-2-deoxy-(1-4)-α-D-glucopyranose residues (D-glucosamine units) with no or a small amount of N-acetyl-D-glucosamine units, is characterised by its strong affinity toward transition metal (Fig. 1b) [9]. This biopolymer, which is derived mainly from the shells of crustaceans is produced in considerable amounts each year. Chitosan has already been exploited in heterogeneous catalysis for the reduction of nitro-aromatic derivatives [10], cyclopropanation of olefins [11], suzuki and heck reactions [12].
Supported ionic liquid phase catalysts (SILC) based on alginate and chitosan supports were prepared as follow: a thin film of ionic liquid containing the homogeneous catalyst was immobilized on the surface of the biopolymer support (Fig. 2).
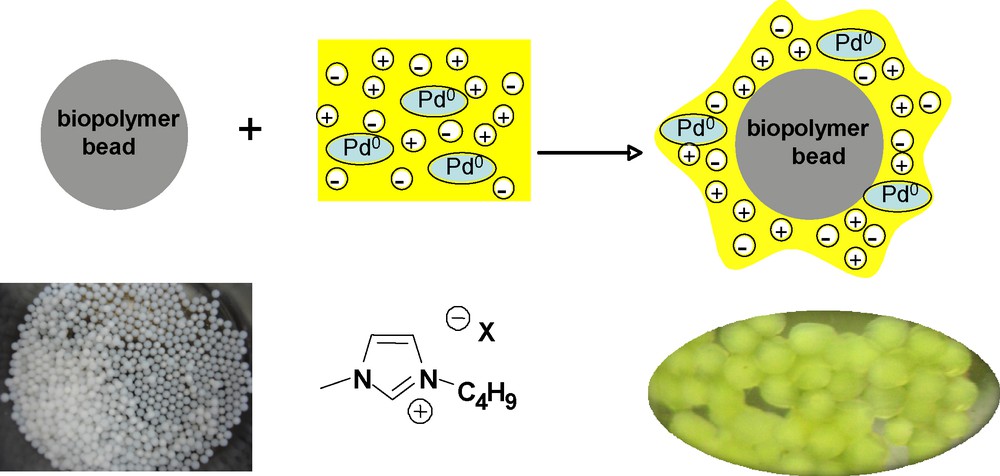
Biopolymer-SILC system.
Typically, the catalytic materials were prepared by impregnation (physisorption) of lyophilized biopolymer beads by a [bmim][BF4] phase containing 0.05 equivalent of the palladium catalyst (Pd(OAc)2) and 0.2 equivalent of phosphine ligand with a ratio biopolymer weight/IL volume of 50 mg/0.3 mL and 100 mg/0.5 mL for alginate and chitosan, respectively. The amphiphilic nature of the polysaccharides presumably accounts for the great immobilization of the ionic liquid phase. The ionic ligand TPPTS (P(m-C6H4SO3Na)3) was selected in order to favour the anchoring of the catalyst in the ionic liquid phase. After stirring at room temperature for 30 minutes, the biopolymer-SILC was ready to be used in the palladium catalysed allylic substitution. Alginate and chitosan based catalytic materials were then evaluated in two important palladium catalysed reactions allowing the formation of C-N and C-C bonds.
3.1 C-N bond formation
The model reaction, selected for the C-N bond formation, involved (E)-1,3-diphenyl-3-acetoxyprop-1-ene 1 as the electrophilic moiety and morpholine as nucleophile (Scheme 1). The reactions were conducted with both support under optimal conditions previously determined for chitosan-supported ionic liquid phase catalysts [4].
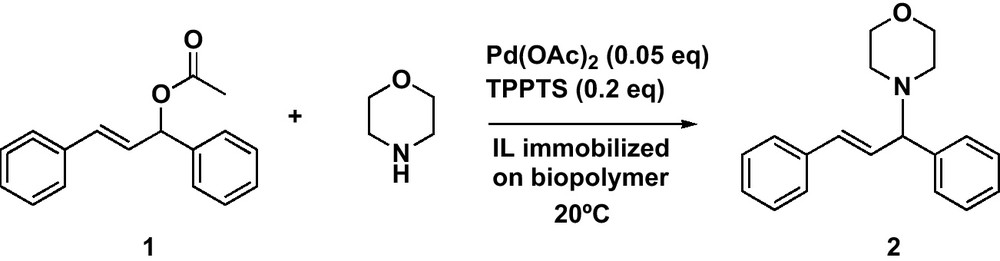
Palladium catalysed allylic substitution: the amination reaction.
The expected allylic amine 2 was obtained in good yields under mild conditions, when using these new catalytic materials whatever the support used, alginate (89%, Table 1, entry 1) or chitosan (91%, Table 1, entry 2). The reaction was completed in less than 30 min with the catalytic material based on alginate and less than 1 hour for that based on chitosan. It is worth noting that in the absence of the phosphine ligand (TPPTS), the reaction was unsuccessful whatever the support, indicating that the complexation of the Pd to the biopolymer either did not occur or led to an inactive organometallic complex in both cases. These preliminary results compare satisfactorily to those obtained under similar conditions in monophasic [bmim][BF4] (92% yield in 30 min), indicating that the biopolymer support does not limit the activity of the catalyst.
Allylic substitution between 1 and morpholine carried out with biopolymer-SILC.
| Support | Time (min) | Conversion (%) | Isolated yield (%) | |
| 1 | Alginate beads | 30 | 100 | 89 |
| 2 | Chitosan beads | 60 | 100 | 91 |
One of the main advantages of using the biopolymer supported ionic liquid phase compared to the reactions performed in pure ionic liquid is the easy product/catalyst separation. By using the biopolymer-supported [bmim][BF4] catalyst, less than one half of the amount of ether required for a complete extraction in pure IL was used. Recovering and re-usability of the catalyst are the other main advantages of these new catalytic materials. Recycling tests showed that the alginate-SILC, could be reused at least for 6 cycles without any loss of activity (conversion > 98%). However, in the 6th cycle, alteration of the beads was seen and in the 7th cycle, a leak of ionic liquid from the beads was observed leading to a lower conversion (91% after 30 min instead of 98%). In comparison, with the chitosan-SILC, the recycling was possible 10 times without any loss in activity (> 98% conversion) (Table 2). Although the recycling proved to be slightly superior with chitosan as support, presumably because of the strong affinity of the amino group toward Pd, it can be noted that with both supports, a good anchoring of the catalyst is observed.
Recycling and re-use of biopolymer–SILC in the allylic substitution of 1 with morpholine.
| Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7a | 8 | 9 | 10 |
| Reaction time (h) with alginate-SILCa | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |||
| Conversion (%) with alginate-SILCa | 98 | 98 | 98 | 98 | 98 | 98 | 91 | |||
| Reaction time (h) with chitosan-SILC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Conversion (%) with chitosan-SILC | 98 | 98 | 98 | 98 | 98 | 98 | 98 | 98 | 98 | 98 |
a Reaction was stopped after the 7th run because of the alteration of the alginate beads.
3.2 C-C bond formation
The model reaction, selected for the C-C bond formation, involves (E)-1,3-diphenyl-3-acetoxyprop-1-ene 1 as the electrophilic moiety and the soft carbon nucleophile dimethylmalonate in the presence of the BSA/AcOK couple (Scheme 2).
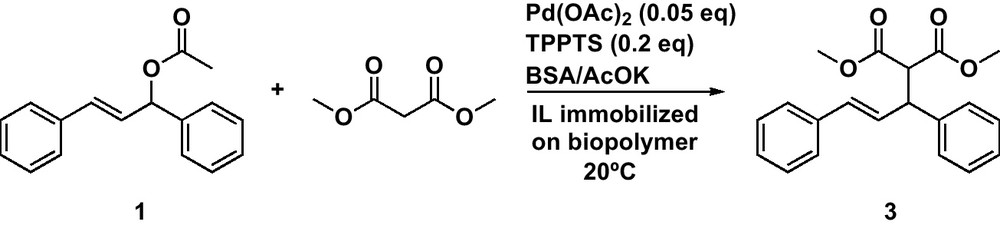
Palladium catalysed allylic substitution.
This quite complex reaction involving a substrate, a reagent and a base to generate the reactive anion is a good choice in order to study the scope and limitation of these new catalysts. Compared to the reaction with morpholine (completed in 1 h), the reaction with dimethylmalonate required prolonged stirring at rt. With chitosan-SILC, the reaction was completed in 18 h, while only 3 h were required for a full conversion with the alginate-SILC (Table 3). Nevertheless, whatever the support, the yields in the expected product are good (96% to 98% after column chromatography [Table 3]). For comparison, the reaction conducted in pure IL was completed in 2 h and afforded an average yield of 98%.
Allylic substitution between 1 and dimethylmalonate carried out with biopolymer-SILC.
| Support | Time (h) | Conversion (%) | Isolated yield (%) | |
| 1 | Alginate beads | 3 | 100 | 96 |
| 2 | Chitosan beads | 18 | 100 | 98 |
With alginate SILC, the recycling tests were conducted for five cycles without significant loss in activity. However, in the sixth cycle a significant decrease of the catalytic activity was measured (60% conversion after 3 days at 20 °C, Table 4).
Recycling and re-use of biopolymer–SILC catalysts in the allylic substitution of 1 with dimethylmalonate.
| Cycle | 1 | 2 | 3 | 4 | 5 | 6 |
| Reaction time with chitosan-SILC | 18 h | 18 h | 18 h | 4 d | 6 d | – |
| Conversion (%) with chitosan-SILC | > 98 | > 98 | > 98 | 93 | 52 | – |
| Reaction time with alginate-SILC | 3 h | 3 h | 3 h | 3 h | 5 h | 3 d |
| Conversion (%) with alginate-SILC | > 98 | > 98 | > 98 | > 98 | 90 | 60 |
With chitosan-SILC, four cycles could be performed with a high conversion (> 93%) (Table 4), although a decrease in the activity was noticed in the fourth run. Hence, four days at 20 °C were required for a complete conversion instead of 18 h in the three first runs. In the 6th cycle, conversion dropped to 52% after 5 days of reaction (Table 4).
From these experiments we can notice that the nature of the support is not innocent for the activity of the catalyst. As can been seen in both examples, the alginate-SILC proved to be superior to those based on chitosan. Although the reason of the lower activity with chitosan is not clear at the moment, the strong basic character of the support can be invoked since it is well know that the allylic substitution reaction is pH sensitive. Further work is currently under progress in order to support this hypothesis.
4 Conclusion
In this work, we have shown that a catalyst containing an IL phase immobilized on biopolymers can be successfully applied to the palladium catalysed substitution reactions. The main advantages of these new catalytic systems are: the use of a renewable ressource as support, the small amount of ionic liquid which is required, the ease of product extraction, the high activity of the catalytic system and its efficient recycling and reuse. Two polysaccharides supports having different functionalities were tested, chitosan, having amino functions and alginates, having acidic functions. This study emphasized the role of the chemical structure on the catalytic properties of SILC. Hence, alginate-SILC proved to be superior to chitosan ones whatever the reaction, leading to highly efficient catalysts, which have activities similar to those obtained under homogeneous conditions in pure ILs. Influence of the drying process of the alginate support (lyophilisation or scCO2 drying) on the catalytic activity and the recycling of the catalytic materials are currently under way.
Acknowledgements
This work was performed within the interregional networks: RMPP and CRUNCH. We gratefully acknowledge financial support from the “Agence Nationale de la Recherche, projet no ANR-08-CP2D-02”, the “Ministère de la Recherche et des Nouvelles Technologies”, the CNRS (Centre National de la Recherche Scientifique) within the RdR3 network, the “Région Basse-Normandie” and the European Union (FEDER Funds).


