1 Introduction
Pyridyl and pyrazine amide ligands have garnered interest due to their biomimetic potential and their variable ligation modes. In this regard, the interaction of transition metals and peptide groups, CONH, is of special interest as an important construction unit in biology [1]. A large variety of pyridineamide ligands have been synthesized for investigation of their metal-binding properties [2] and for providing models from the standpoint of bioinorganic chemistry [3]. On the other hand, copper(II) is integral to the active site of numerous electron transfers and oxygenase metalloprotein [4].
To study the chemistry of the 2-pyridinecarboxamide moiety, herein, we report the synthesis, molecular and crystal structure and properties of a new copper(II) complex with N-(2-pyridylmethyl)-2-pyrazinecarboxamide (NPyPzCa), Scheme 1. This ligand in the deprotonated form, NPyPzCa−, binds a copper(II) center in a planar tridentate arrangement with two aromatic-N and one carboxamido nitrogen. Electrochemical and thermal properties of this complex have also been examined.
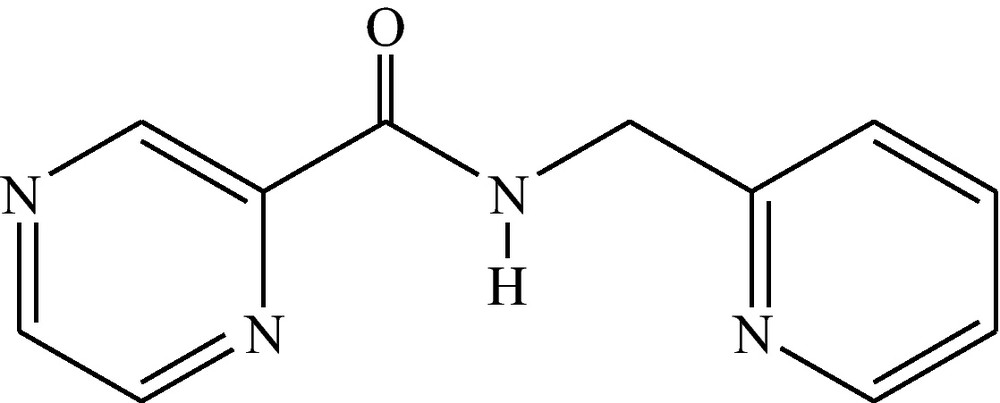
N-(2-pyridylmethyl)-2-pyrazinecarboxamide ligand.
2 Experimental
2.1 Materials and instruments
All reagents and solvents were purchased from chemical sources and were used directly without further purification. UV–vis spectra were recorded on a Shimadzu 2100 spectrometer using a 1 cm path length cell. Infrared spectra (4000–450 cm−1) of solid samples were taken as 1% dispersion in KBr pellets using a Shimadzu-470 spectrometer. Elemental analysis was performed using a Heraeus CHN-O Rapid analyzer. Cyclic voltammetery was performed using a Metrohm computerized voltammetric analyzer model 747 VA stand. Three electrodes were utilized in this system, a platinum disk-working electrode (RDE), a platinum wire auxiliary electrode and Ag/AgCl reference electrode. The platinum disk-working electrode was manually cleaned with 1-μm diamond polish prior to each scan. The supporting electrolyte, 0.1 M tetrabutylammonium perchlorate ([nBu4N][ClO4]), was recrystallized twice from ethanol/water (1/1) and vacuum-dried at 110 °C overnight. Thermal analysis of the title compound was performed on a Rheometric Scientific STA-1500 with a heating rate of 10 °C/min.
2.2 Synthesis of [Cu(NPyPzCa)(NO3)(DMSO)]
Cu(NO3)2·2.5H2O (0.01 mmol) was dissolved in ethanol (10 ml) and mixed with the NPyPzCa ligand [5] (0.02 mol) dissolved in 10 ml DMSO. The mixture was stirred at 80–85 °C for 3 h. The solvent was removed and a blue precipitate was obtained. The vapor diffusion of diethyl ether into the DMSO solution of the purified blue product leads to the formation of crystals of [Cu(NPyPzCa)(NO3)(DMSO)] (1), yield 70%, 0.29 g IR(KBr, cm−1) selected bands: (718, 763, 865, 921, 1009, 1044, 1307, 1427, 1473, 1643 and 1669): UV–vis (λ): 560–650 nm. Anal. Calcd for C13H15Cu1N5O5S1 (%): C, 37.45; H, 3.63; N, 16.80. Found: C, 37.33; H, 3.54; N, 16.69.
2.3 X-ray crystallography
The X-ray diffraction measurements were made on a STOE IPDS-II diffractometer with graphite monochromated Mo-Kα radiation. Data was collected at a temperature of 293(2) K in a series of ω scans in 1° oscillations and integrated using the Stoe X-AREA software package [6]. A numerical absorption correction was applied using X-RED [7] and X-SHAPE [8] softwares. The structure was solved by direct methods and subsequent difference Fourier maps and then refined on F2 by a full-matrix least-squares procedure using anisotropic displacement parameters [9]. All hydrogen atoms were located in a difference Fourier map and then refined isotropically. Atomic factors are from the International Tables for X-ray Crystallography [10]. All refinements were performed using the X-STEP32 crystallographic software package [11].
2.4 Computational procedure
All calculations have been carried out by using the Gaussian-98 (R-A.9) software [12]. In order to obtain local minima geometries of the title molecule in the ground state (in vacuo), BP86/6-311+G(d) and B3LYP/6-311+G(d) have been employed. All initial geometries were assumed as atomic coordinates obtained from the X-ray analysis as initial geometry. Then, harmonic vibrational frequencies were computed at both levels. The Gaussian program defaults were used as appropriate optimization criteria.
3 Results and discussion
Compound 1 was obtained from the reaction of one equivalent of Cu(NO3)2·2.5H2O with one equivalent of N-(2-pyridylmethyl)-2-pyrazinecarboxamide [5] in ethanol/DMSO at 80–85 °C during 3 h, Eq. (1). The reaction yield was quantitative and the resulting compound was insoluble in common organic solvents except for DMSO.
| (1) |
The infrared spectrum of the title compound exhibits the band expected for 2-pyrazinecarboxamide ligand coordinated to the copper(II) ion, which is in agreement with X-ray crystal structure. Coordination of carboxamido nitrogen to copper(II) is evident from the shift of the carbonyl stretching frequency (υCO) from 1669 to 1643 cm−1. The stretching vibration of υCN was observed at 1473 cm−1. Other important features of the infrared spectrum are the strong bands at 921, 865, 763 and 718 cm−1 due to the aromatic skeleton vibrations of the pyridine and pyrazine rings and the υSO strong bands at 1009 cm−1, indicating the presence of the DMSO molecule coordinated from the oxygen atom. Three strong NO stretching bands, as expected for NO3− with C2v symmetry, are observed at 1427, 1307 and 1044 cm−1. The blue color for this complex arises from a broad d–d transition band in the 560–650 nm regions.
The molecule as well as the atom numbering scheme of the title copper(II) complex is illustrated in Fig. 1. Crystallographic data and parameters are summarized in Table 1. Selected experimental and optimized bond lengths (Å) and angles (°) with their standard deviations are given in Table 2. The results indicate that the complex is a local minimum, without any imaginary frequency. The calculations corroborate well to the available experimental data, yet the geometrical values computed at BP86/6-311+G(d) level seem to better agree with the experimental ones. The asymmetric unit of this compound contains two independent copper(II) complexes. The copper(II) center is in a square pyramidal geometry with the apical position occupied by a molecule of DMSO. In the equatorial positions, NPyPzCa− acts as a tridentate ligand and an oxygen atom from nitrate anion, trans to the carboxamide nitrogen, completes the coordination sphere in the square plane. Interestingly, the coordination of the NO3− ion is stabilized by a short contact between one of the non-coordinated oxygens of NO3− and copper ion. The copper(II)-ONO2 distances are 2.024(8) and 2.030(7) Å for coordinated and 2.650(7) and 2.677(8) Å for the non-coordinated one. It is evident from Fig. 1 that the second oxygen atom of the nitrate ligands is positioned towards the Cu and the distance of this CuO is a little larger than the bond distance observed for normal CuO. Therefore, we conclude that nitrate acts as a semi-bidentate ligand. The Cu(II)Namido (1.920(10) and 1.912(10) Å), Cu(II)NPy (2.007(9) and 1.993(9) Å) and Cu(II)NPz (2.026(9) and 1.996(9) Å) distances in this complex are comparable to analogous distances in other known complexes [13].

The ORTEP diagram of the asymmetric unit of the copper complex. Thermal ellipsoids are at 30% probability level.
Crystallographic and structure refinements data of 1.
| Formula | C13H15Cu1N5O5S1 |
| Formula weight | 416.92 |
| Temperature (K) | 293(2) |
| Wavelength, λ (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Crystal size (mm) | 0.50 × 0.50 × 0.30 |
| a (Å) | 10.829(10) |
| b (Å) | 20.646(2) |
| c (Å) | 15.017(2) |
| β (°) | 100.88(1) |
| Volume (Å3) | 3297.1(7) |
| Z | 8 |
| Density (calc.) (g cm−1) | 1.680 |
| θ ranges for data collection | 1.70–28.02 |
| F(000) | 1704 |
| Absorption coefficient | 1.488 |
| Index ranges | −14 ≤ h ≤ 14 |
| −27 ≤ k ≤ 27 | |
| −19 ≤ l ≤ 17 | |
| Data collected | 33606 |
| Unique data (Rint) | 7881, (0.0955) |
| Parameters, restrains | 455, 0 |
| Final R1, wR2a (Obs. data) | 0.0814, 0.1806 |
| Final R1, wR2a (All data) | 0.0871, 0.1925 |
| Goodness of fit on F2 (S) | 1.356 |
| Largest diff peak and hole, e (Å−3) | 0.975, −0.894 |
a R1 = Σ||Fo| − |Fc||/Σ|Fo|, wR2 = [Σ(w(Fo2−Fc2)2)/Σw(Fo2)2]1/2.
Selected experimental and optimized bond lengths (Å) and angles (°) for 1.
| Bond lengths (X-ray/BP86+G/B3LYP+G) | Bond angles (X-ray/BP86+G/B3LYP+G) | ||
| Cu(1)-N(1) | 2.007(9)/2.04558/2.06123 | N(1)-Cu(1)-N(2) | 82.5(4)/81.1/80.7 |
| Cu(1)-N(2) | 1.920(10)/1.9357/1.92782 | N(1)-Cu(1)-N(3) | 163.6(4)/160.6/159.8 |
| Cu(1)-N(3) | 2.026(9)/2.07052/2.09032 | N(2)-Cu(1)-N(3) | 81.6(4)/80.8/81.0 |
| Cu(1)-O(2) | 2.024(8)/1.99395/1.99046 | N(1)-Cu(1)-O(2) | 97.7(3)/99.1/98.4 |
| Cu(1)-O(5) | 2.299(8)/2.36021/2.3522 | N(1)-Cu(1)-O(5) | 93.0(3)/93.8/94.3 |
| Cu(2)-N(6) | 1.993(9) | N(2)-Cu(1)-O(2) | 164.1(4)/175.1/169.5 |
| Cu(2)-N(7) | 1.912(10) | N(2)-Cu(1)-O(5) | 111.0(3)/101.4/115.3 |
| Cu(2)-N(8) | 1.996(9) | N(3)-Cu(1)-O(2) | 96.5(3)/98.1/98.4 |
| Cu(2)-O(7) | 2.030(7) | N(3)-Cu(1)-O(5) | 96.2(3)/96.7/97.1 |
| Cu(2)-O(10) | 2.307(8) | O(2)-Cu(1)-O(5) | 85.0(3)/83.4/81.7 |
| N(6)-Cu(2)-N(7) | 82.2(4) | ||
| N(6)-Cu(2)-N(8) | 164.3(4) | ||
| N(7)-Cu(2)-N(8) | 82.2(4) | ||
| N(6)-Cu(2)-O(7) | 98.0(3) | ||
| N(6)-Cu(2)-O(10) | 96.3(4) | ||
| N(7)-Cu(2)-O(7) | 164.6(4) | ||
| N(7)-Cu(2)-O(10) | 109.8(4) | ||
| N(8)-Cu(2)-O(7) | 96.6(3) | ||
| N(8)-Cu(2)-O(10) | 90.4(4) | ||
| Torsion angles (X-ray/BP86+G/B3LYP+G) | |||
| N(1)-Cu(1)-O(2)-N(5) | −88.6(7)/−90.5/−91.1 | ||
| N(2)-Cu(1)-O(2)-N(5) | 1.1(9)/2.5/1.8 | ||
| N(3)-Cu(1)-O(2)-N(5) | 83.3(7)/80.7/79.6 | ||
| O(5)-Cu(1)-O(2)-N(5) | 179.0(7)/176.6/180.5 | ||
| N(6)-Cu(2)-O(7)-N(10) | 81.2(6) | ||
| N(7)-Cu(2)-O(7)-N(10) | −8.4(2) | ||
| N(8)-Cu(2)-O(7)-N(10) | −93.0(6) | ||
| O(10)-Cu(2)-O(7)-N(10) | 177.0(6) |
Considerable strain exists in the coordination plane around the copper(II) center due to the bite (164°) of the NPyNamidoNPz portion of the NPyPzCa− ligand frame. The angle between the basal equatorial coordination plane and the heterocyclic rings are 11.05(8) and 8.16(6)° for pyridine and 9.31(7) and 9.29(6)° for the pyrazine ring. These angles have remarkable structural significance because they enable some weak intermolecular interactions of the type C(atom)-H…O(DMSO) and C(atom)-H…O(NO3−), as Table 3 and Fig. 2 reveal. The presence of C-H…O hydrogen bonds in liquids has found increasing interest [14] due to a significant role in a wide variety of chemical phenomena, from crystal packing and supramolecular designs to structure-activity relationships in biological systems [15].
Geometry of the hydrogen bonds and C-H…π(Py ring) interaction of complex 1 (Å and°).
| D-H…A | d(D-H) (Å) | d(H…A) (Å) | d(D…A) (Å) | (D-H…A) (°) |
| C(atom)-H…O(DMSO) | ||||
| C4-H4…O10a | 0.9300 | 2.5800 | 3.440(17) | 153.0 |
| C22-H22…O5b | 0.9300 | 2.4600 | 3.334(17) | 157.0 |
| C(atom)-H…O(NO3−) | ||||
| C9-H9…O8b | 0.9300 | 2.3800 | 3.254(17) | 157.0 |
| C11-H11…O9c | 0.9300 | 2.4200 | 3.082(15) | 128.0 |
| C14-H14…O3d | 0.9300 | 2.3800 | 2.985(16) | 122.0 |
| C17-H17…O4a | 0.9300 | 2.4000 | 3.273(16) | 156.0 |
| C(DMSO)-H…O(carbonyl) | ||||
| C25-H25…O1e | 0.9600 | 2.4300 | 3.173(18) | 134.0 |
| C13-H13…O6f | 0.9600 | 2.4800 | 3.224(16) | 134.0 |
| C(pyridine)-H…N(pyrazine) | ||||
| C16-H16…N9g | 0.9300 | 2.5800 | 3.777(19) | 145.0 |
| C-H…π(Py ring) | ||||
| C6-H6A…Py(N6/C14-C18)a | – | 2.850 | – | 143.0 |
| C19-H19B…Py(N1/C1-C5)a | – | 2.741 | – | 145.0 |
a –X, 1 − Y, 1 − Z.
b 1 − X, 1 − Y, 1 − Z.
c X, 1/2 − Y, 1/2 + Z.
d X, 1/2 − Y, −1/2 + Z.
e X, −1 + Y, Z.
f X, 1 − Y, 1 − Z.
g −1 + X, 1/2 − Y, −1/2 + Z.
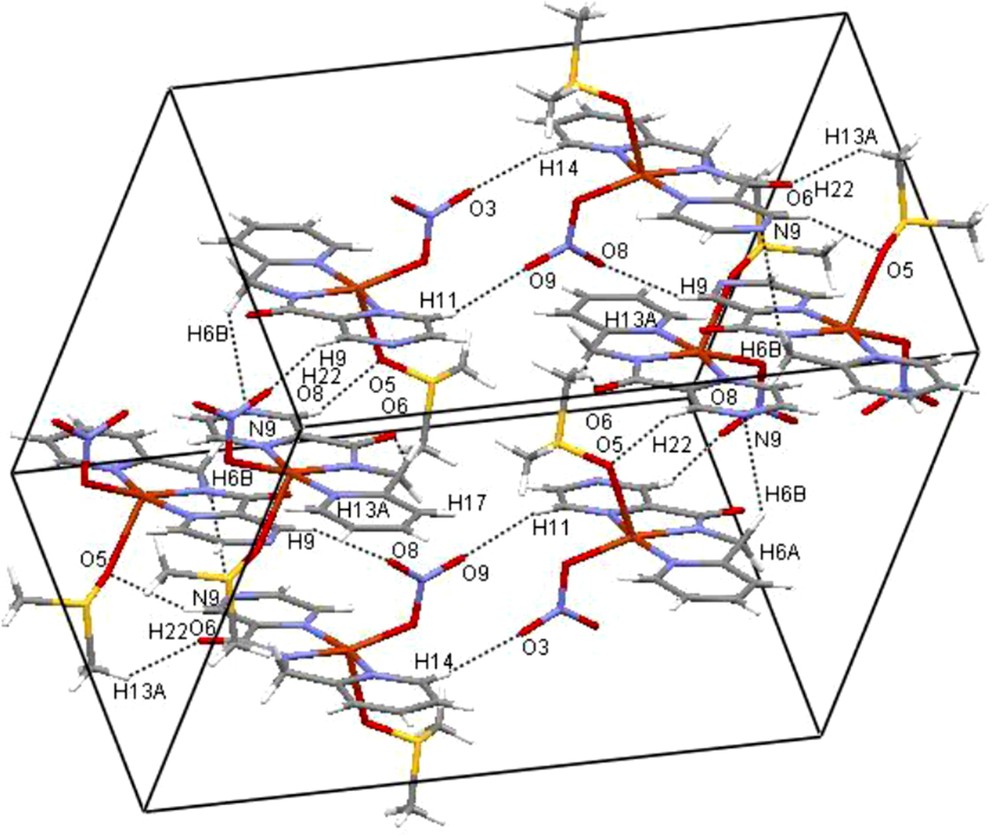
A view of N-(2-pyridylmethyl)-2-pyrazinecarboxamide copper(II) complex, showing two adjacent molecules linked by intermolecular hydrogen bonds (dashed lines).
There is also some C(DMSO)-H…O(carboxamide carbonyl) and one C(pyridine)-H…N(pyrazine), Table 3, that seem to be effective in the stabilization of the crystal packing. The NO3− plane is almost perpendicular to the basal coordination plane and the angles are 87.1(1)° for molecule 1 (containing Cu(1)) and 84.7(2)° for molecule 2 (containing Cu(2)). No π,π-stacking interactions are operative but there are some CH…π(Py ring) interactions, Table 3, affecting the lattice structure of this complex.
Electrochemical behavior of this copper(II) complex has been studied in dry DMF in the potential range +0.40 to −0.20 V versus SCE, Fig. 3. The most interesting observation in the positive potential region appears to be a well defined redox couple for which the anodic peak potential corresponds to the oxidation of Cu(II)(3d9) to Cu(III)(3d8) and the cathodic counterpart is due to the reduction of Cu(III) [16]. The oxidation waves at +0.35 V is close to that observed for 1,2-bis(pyridine-2-carboxamide)benzene copper(II) [17] and is assigned as a quasi-reversible one electron Cu(III/II) process.
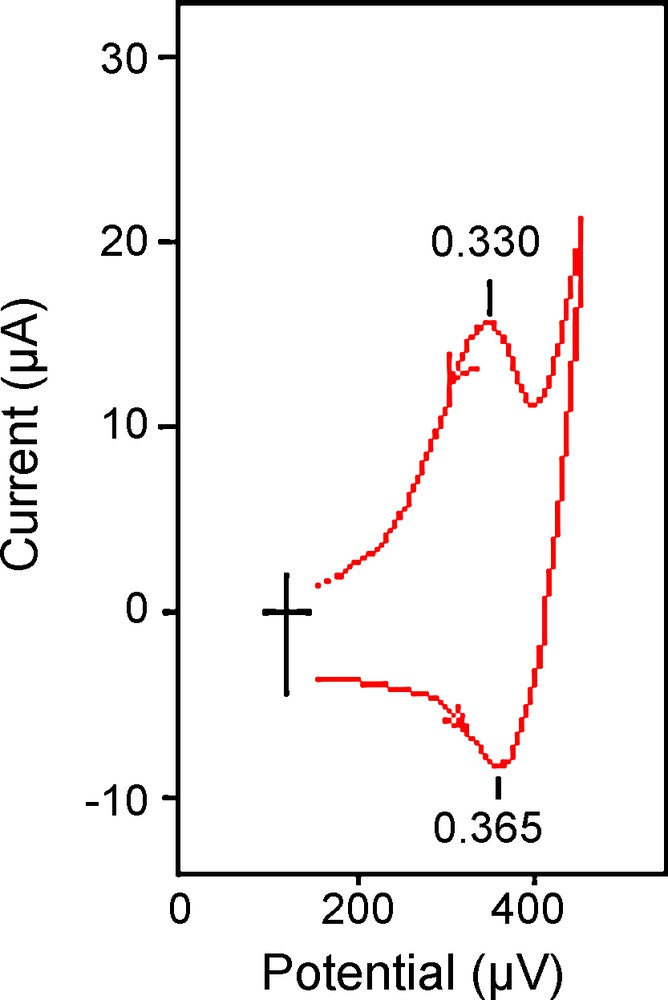
Cyclic voltammogram (scan rate: 100 mV/s) of 10−3 mol dm−3 DMF (∼0.1 mol dm−3 in [nBu4N][ClO4]) solution of 1.
The thermal behavior of this complex was studied by thermogravimetric analysis (TGA) under a nitrogen atmosphere with the heating rate of 10 °C/min. The results show that this complex is so stable that no obvious decomposition was observed below 220 °C. In the temperature range 225–260 °C, the thermogram showed a mass loss corresponding to NO3− and DMSO coordinated species (observed = 33.1%, calculated = 33.6%), and then from 260 to 440 °C, gradually decomposed to form an amorphous phase with the loss of the 2-pyridinecarboxamide ligand (observed = 49.7%, calculated = 51.1%).
Acknowledgement
We are grateful to Prof. S.W. Ng for providing us the software (G98W suite of programs) and hardware (machine time) facilities and also making us available the opportunity to access some new features of Gaussian products. We would like to thank the Graduate Study Councils of Shahid Beheshti University for financial support.


